Abstract
Purpose
In contrast to penetrating keratoplasty (PK), the donor cornea in lamellar keratoplasty (LK) remains separated from the host aqueous humor. There is debate about relative merits of each approach, but experimental comparisons have never been performed in animal models. Therefore, we developed a murine LK model.
Methods
For allogeneic PK and LK surgeries, corneas of C57BL/6 mice were transplanted to BALB/c mice, assessed by slit lamp, and scored for opacity, edema, and neovascularization up to 46 d post-transplantation. Additional PK or LK surgeries were performed for histological assessment.
Results
Graft rejection rate was significantly less in LK vs. PK (69.2% vs. 100%), as was neovascularization (84.6% vs. 100%). In LK, inflammatory cells infiltrated primarily the button; in PK, heavier infiltration was observed throughout the cornea.
Conclusions
We demonstrate the feasibility of LK in mice and present data suggesting that the inflammatory response in LK differs from that in PK.
Keywords: cornea, model, mouse, transplantation
INTRODUCTION
Corneal transplantation is the most common solid tissue transplantation procedure in the world. It can be performed using full-thickness donor corneal buttons (penetrating keratoplasty or PK) or partial-thickness buttons (lamellar keratoplasty or LK). In PK, the posterior part of the donor cornea forms a new wall of the anterior chamber and contacts the host aqueous humor, whereas in LK, the donor cornea remains separated from the aqueous humor. Although the aqueous humor plays an important role in the maintenance of immune privilege, and the aqueous humor directly contacts the graft in PK, PKs tend to be rejected at higher rates than LK1.
The high surgical success of corneal transplantation can be attributed in part to immune privilege of the eye. Although HLA matching is not normally performed and systemic immunosuppressive drugs are not routinely used in humans, this procedure results in approximately 90% survival rate in uncomplicated and low-risk grafts2. Several factors may contribute to this high success rate including; (1) absence of blood and lymphatic vessels in the cornea3,4; (2) low expression of major histocompatibility (MHC) class I and II antigens on corneal cells5-8; (3) expression of complement regulatory proteins in the aqueous humor and on corneal cells9,10; (4) relatively high concentrations of immunosuppressive factors such as transforming growth factor beta and alpha melanocyte stimulating hormone in aqueous humor11,12; (5) expression of apoptosis-inducing factors such as FasL on corneal endothelium10,13,14 and (6) anterior chamber-associated immune deviation (ACAID)5-8,15-17. The roles of these factors, especially factors 3 through 6, on allograft success would be different for PK and LK, given that in LK the donor button does not directly contact the aqueous humor in the anterior chamber.
Experimental animal models of corneal transplantation have proven invaluable in advancing the understanding of immunological mechanisms involved in graft rejection. Murine models are particularly useful given the availability of transgenic animals that allow for the study of individual factors that are important in immunological processes. However, most studies of corneal transplantation in the mouse have utilized PK. There has been one study of murine interlamellar keratoplasty by Lau et al.18 in which a corneal pocket is established in a host cornea that is left in situ. This, however, does not mimic standard clinical practice.
The transplantation of individual layers of the cornea has been investigated in the rabbit model19,20, but to our knowledge, there are no other described studies of LK in the mouse. Therefore, we sought to establish a model of LK in the mouse which was used to compare the natural clinical course and histological features with the conventional PK model.
MATERIALS AND METHODS
Mice
Inbred female C57BL/6 mice (donor) and BALB/c mice (recipient) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were age 8 to 10 weeks at the time of transplantation. All animal procedures were performed in accordance with institutional guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Animals were housed in the animal care facilities of the Oregon Health & Science University and received food and water ad libitum.
Penetrating keratoplasty (PK) procedure
Penetrating keratoplasties were performed with minor modification from previously published methods21. In summary, Provisc (Alcon Laboratories, Fort Worth, TX) was injected into the anterior chamber of the eye of an anesthetized donor.
A 2 mm trephine (Storz, St. Louis, MO) was used to mark the cornea. A 25G needle was used to make an initial incision at the trephination site and Vannas scissors were used to cut the button out. The button was kept in chilled ophthalmic balanced saline solution (BSS) (Alcon) while the recipient bed was prepared. The recipient was anesthetized by inhalation of 2% isoflurane in oxygen. The cornea was marked with a 1.5 mm curette. After a small incision was made with a 25G needle, Vannas scissors were used to cut out a corneal disk, which was discarded. The donor button was then sutured in place first with 4 interrupted sutures (11-0 nylon, Alcon) followed by a running suture.
Lamellar keratoplasty (LK) procedure
To prepare the donor button, the animal was killed by CO2 inhalation; the anterior segment (cornea and iris) was removed by cutting just behind the limbus with scissors and placed with the endothelium facing up. The iris was removed with forceps and BSS was used to wash away tissue fragments. The endothelium was removed by scraping with a sterile swab after the iris was removed. The corneal button was punched out under the hydration of BSS with a 2mm trephine (Storz, St. Louis, MO) and then kept in chilled BSS while the recipient was prepared. Recipients were anesthetized by inhalation of isoflurane (2% in oxygen). The cornea was marked with a 1.5 mm curette, and a 25G needle was used to create an initial entry incision. Forceps were used to hold the lamellar button while the sharp edge of a 25G needle was used to separate the corneal lamellae by gradual lateral sweeping motion of the needle until the 1.5mm diameter mark was reached in all directions. Vannas scissors were then used to cut out a button that was approximately ¾ corneal thickness. The donor button was then placed onto the recipient bed with the donor epithelium facing upwards. The graft was sutured in place using four interrupted sutures followed by running 11-0 nylon sutures.
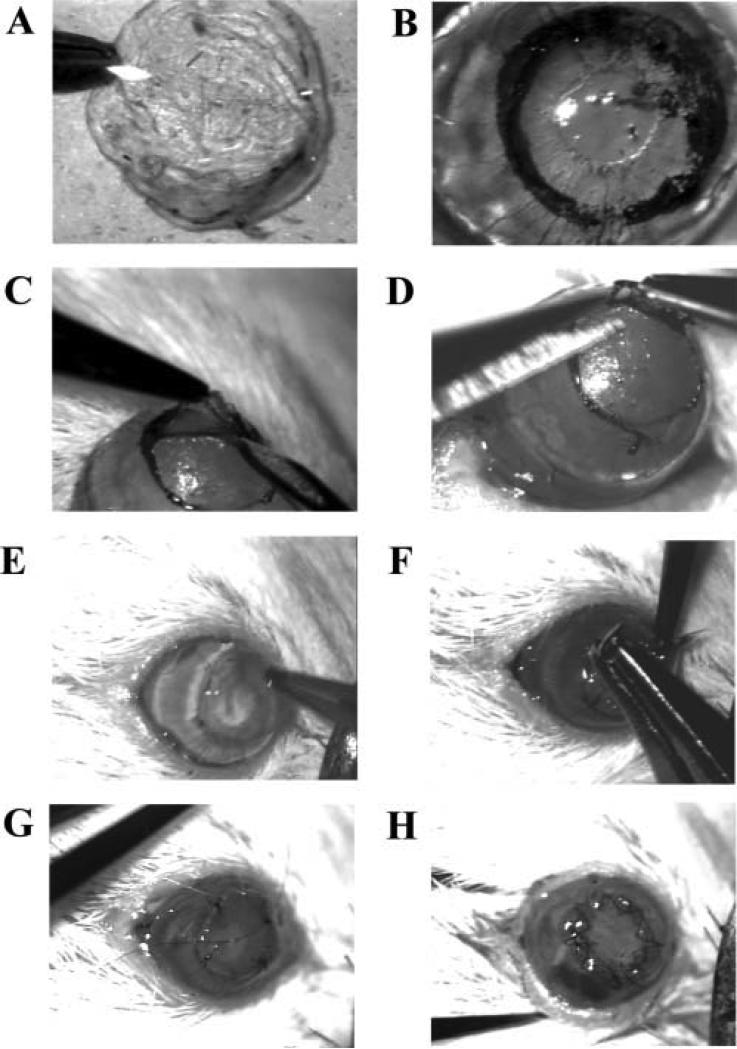
For both LK and PK surgeries, after the running suture was in place, all interrupted sutures were removed. The eyes were then covered prophylactically with 0.3% tobramycin (Akorn, Buffalo Grove, IL). The running sutures were removed 7 days after surgery for all LK and PK. All LK and PK surgeries were performed under the hydration of BSS by the same surgeon (TH).Various steps of this procedure are shown in Figure 1.
Figure 1.
Steps in lamellar keratoplasty surgery. (A) Punch donor corneal button. (B) Mark recipient cornea. (C) Separate lamellae. (D) Cut off lamellar corneal button. (E) Position donor button. (F) Place interrupted sutures. (G) Start running suture. (H) Finished surgery.
Experimental protocol
For comparing LK with PK, 13 LK surgeries and 13 PK surgeries were compared. Both groups were followed clinically by slit lamp examination. Separate surgeries (2 PKs and 2 LKs for each indicated timepoint) were performed for histological examination with standard hematoxylin and eosin staining of paraffin sections.
Follow-up evaluation
After transplantation, graft status was scored every day or every two days until day 46 using slit lamp biomicroscopy for clinical signs of rejection and non-specific inflammation. The central graft with surrounding recipient bed was scored for stromal opacity, corneal edema, and neovascularization (scoring systems summarized in Table 1). Grafts were defined as rejected when the opacity score was 2 or greater and persisted for at least 1 week. After observation, the eye was covered with 0.3% tobramycin solution. Animals were coded so that the graft status could be followed in masked fashion for 50 days.
Table 1.
Scoring scales for graft status
| 0 | 1 | 2 | 3 | 4 | 5 | Reference | |
|---|---|---|---|---|---|---|---|
| Neovascularization | None | Within peripheral half of recipient cornea | Within central half of recipient cornea | At recipient-graft border | Within peripheral half of graft | Within central half of graft | Lau et al. [18] |
| Stromal opacity | Clear | Minimal superficial opacity | Mild deep stromal opacity with pupil margin and visible iris structures | Moderate stromal opacity with only pupil margin visible | Intense stromal opacity with AC visible | Maximal corneal opacity with total obscuration of AC | Sonoda et al. [17] |
| Corneal edema | None | Increased thickness, but less than 1.5× normal thickness | Equal or greater than 1.5× but less than 2× normal thickness | Equal to or greater than 2× but less than 3× normal thickness | Equal to or greater than 3× normal thickness without epithelial bullae | Equal to or greater than 3× normal thickness with epithelial bullae | Sonoda et al. [17] |
Histology
Eyes were enucleated whole at various times as indicated in the Results and fixed in 10% neutral-buffered formalin (NBF) for 24h at 4°C, then automatically dehydrated and infiltrated with paraffin (Citadel 2000, Shandon, Cheshire, England). Specimens were embedded in paraffin and stored at room temperature. Sections (5 μm) were cut using a Leica microtome (American Optical Company, New York, USA) and stained with hematoxylin and eosin.
Statistics
The Mann-Whitney test was used to determine P values in the study of clinical course of graft status. P values less than 0.05 were considered significant.
RESULTS
Clinical course of graft status
We compared the clinical course of graft status of LK with PK. Of seventeen LKs performed, 23.5% (4/17) suffered from complications (two of intraoperative accidental penetration of the graft bed, two of postoperative hyphema). Of eighteen PKs performed, 27.8% (5/18) resulted in complications (two of postoperative cataract, two of hyphema, and one of wound dehiscence). Any eye that suffered complications (e.g., from technical flaws during surgeries) was omitted from follow-up study. During the early stages post-transplantation (before Day 3), all lamellar grafts were clear, but 12 penetrating grafts (12/13, 92.3 %) developed stromal opacity and stromal edema to different extents. Stromal edema was transient and disappeared within 3 to 5 days after the operation. Figure 2 shows slit lamp photographs of clinical follow-up of PKs and LKs.
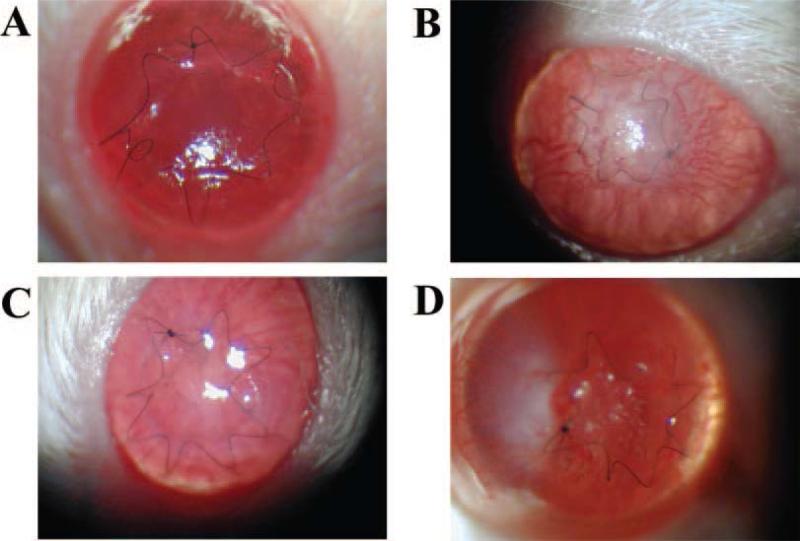
Figure 2.
Slit lamp biomicroscopy following surgery. Clear lamellar graft (day 2 after transplantation) (A); Lamellar graft undergoing rejection (day 8 after transplantation) (B); Clear penetrating graft (day 2 after transplantation) (C); Penetrating graft undergoing rejection (day 7 after transplantation) (D).
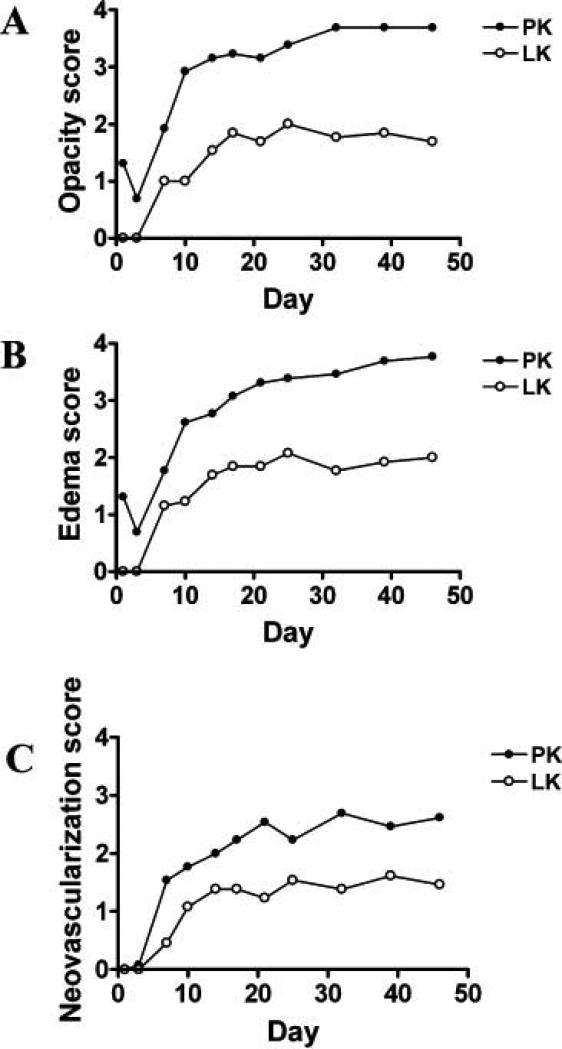
The severity of several clinical parameters (including graft opacity, stromal edema, and neovascularization) were quantified by assigning clinical scores (Figure 3). Stromal opacity associated with PK was significantly greater than with LK at all timepoints post-surgery (Figure 3A). With LK, 69.2% of grafted corneas were rejected on average 10.8 days after transplantation, while some survived beyond the follow-up period. In contrast, all corneal grafts (100%) were rejected by 10 days in PK, with a mean survival time of 7.7 days. Stromal edema was significantly greater after PK compared to LK at all time points except for day 7 (p=0.174) (Figure 3B). Neovascularization was significantly greater in PK compared to LK at all time points after day 17. Neovascularization was observed in 84.6% of LK grafts on average 10.6 days after transplantation and 100% of PK grafts on average 7.4 days after transplantation (p=0.006) (Figure 3C).
Figure 3.
Clinical scores following penetrating or lamellar transplantation. Stromal opacity scores of PKs increased steeply early after surgery (days 3-10) before reaching a plateau (after day 32), whereas stromal opacity scores of LKs increased steadily (days 7-25) then subsided slowly (after day 25) (A). After day 21, mean stromal edema scores of LKs reached a plateau, while they continued to increase in PK; (B). Early after grafting (day 21 for PK, day 14 for LK), neovascularization increased quickly. After day 32 for PK or day 25 for LK, neovascularization reached a plateau (C).
Histological observation of transplanted corneas
For the lamellar keratoplasty surgery, a recipient bed that was ¾ total corneal thickness (Figure 4) was created. By day 26 post-grafting, the separation between donor button and recipient bed was still obvious even when the graft was accepted (Figure 5A), but appeared similar to normal cornea by day 40 when the accepted grafted lamellar buttons were clear, with intact structure and normal epithelial and endothelial cell morphology (Figure 5B). The histological features of rejection were distinct during different phases of the rejection process. In the early rejection phase after lamellar grafting, an inflammatory response was mainly limited to within the lamellar button. Epithelial proliferation, cellular infiltration, and neovascularization could be seen, but the stromal structure and morphology of keratocytes appeared to be intact. This response progressed to feature heavy mixed cellular infiltration and neovascularization within the buttons accompanied by destruction of the button stroma and loss of endothelial cells. However, the recipient epithelium remained intact. In the latter stage of graft rejection of PK, rejected lamellar buttons were characterized by destruction of button stroma and disappearance of the graft keratocytes. Usually minimal cellular infiltration was detected in the graft (Figure 6).
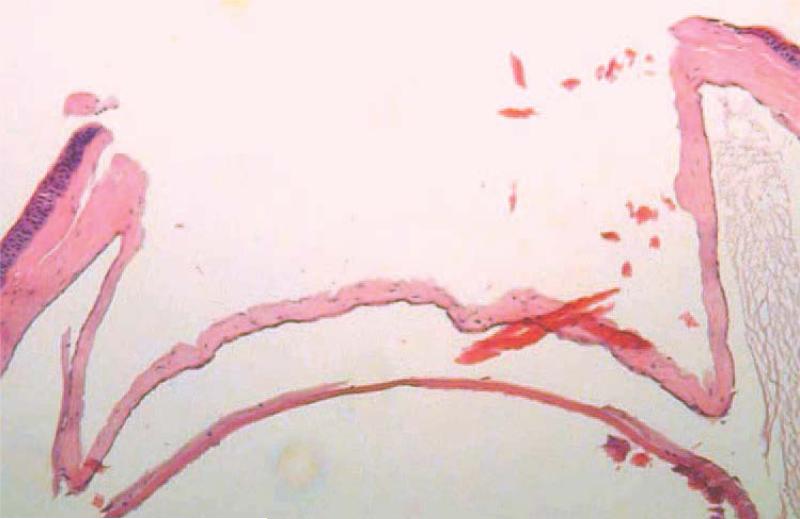
Figure 4.
Lamellar depth of recipient bed. The prepared recipient bed was approximately 3/4 or more of the total corneal thickness.
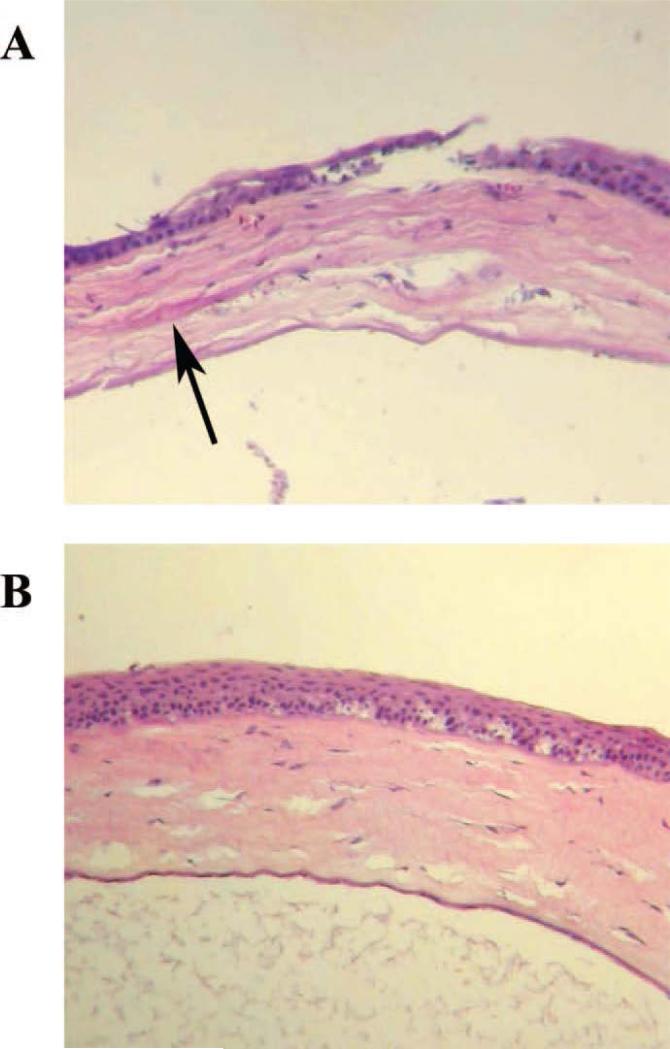
Figure 5.
Accepted LK grafts. A graft in which the separation between button and recipient bed (arrow) remained distinct (day 26 after transplantation) (A); An accepted graft with intact cell and tissue structure (day 40 after transplantation) (B).
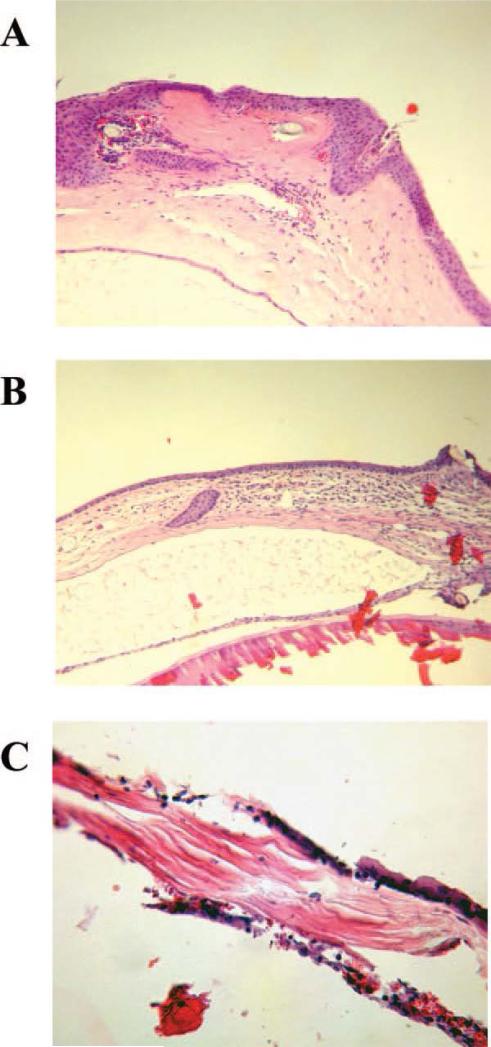
Figure 6.
LK grafts undergoing rejection at various times after transplantation. LK graft undergoing rejection 2 days post graft (A); LK graft undergoing rejection 9 days after grafting (B); LK graft undergoing rejection 36 days after surgery (C).
Epithelial proliferation and cysts were sometimes observed in the donor button in LK, but not in PK (Figure 7). Interlamellar cysts were usually located in the center of the button and histological observation suggested they resulted from epithelium, not endothelium.
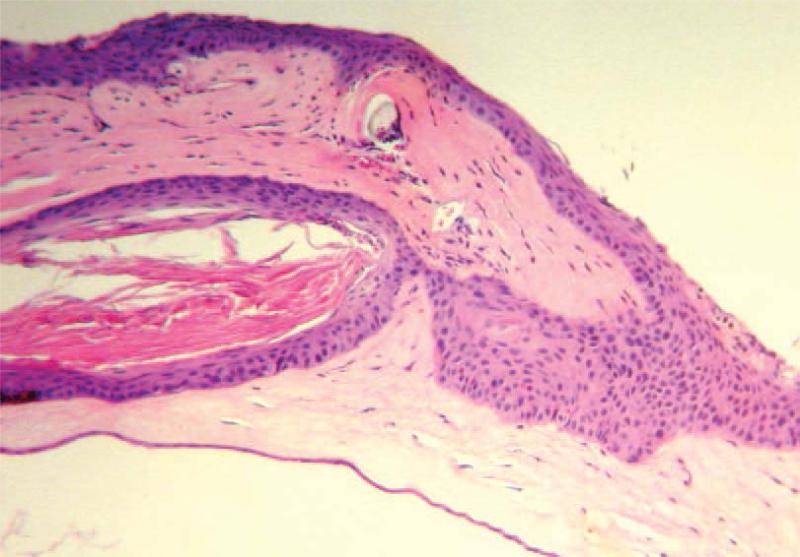
Figure 7.
Epithelial cell ingrowth through the incision. Heavy epithelial proliferation could form a cellular cyst, with the cavity full of material stained by eosin. This was usually accompanied by minimal infiltration and neovascularization, but not necessarily with any clinical rejection benchmark. An LK graft on day 18 post-transplantation is shown.
In contrast to LK, the rejection response in PK involved the full thickness of grafted corneas, especially epithelium and endothelium. Heavy mixed cellular accumulation consisting of mononuclear cells, cells with a more dendriform morphology that could be dendritic cells or keratocytes, and polymorphonuclear cells was detected in both epithelium and endothelium and moderate cellular infiltration was observed in stroma. Usually this was accompanied by neovascularization (Figure 8).
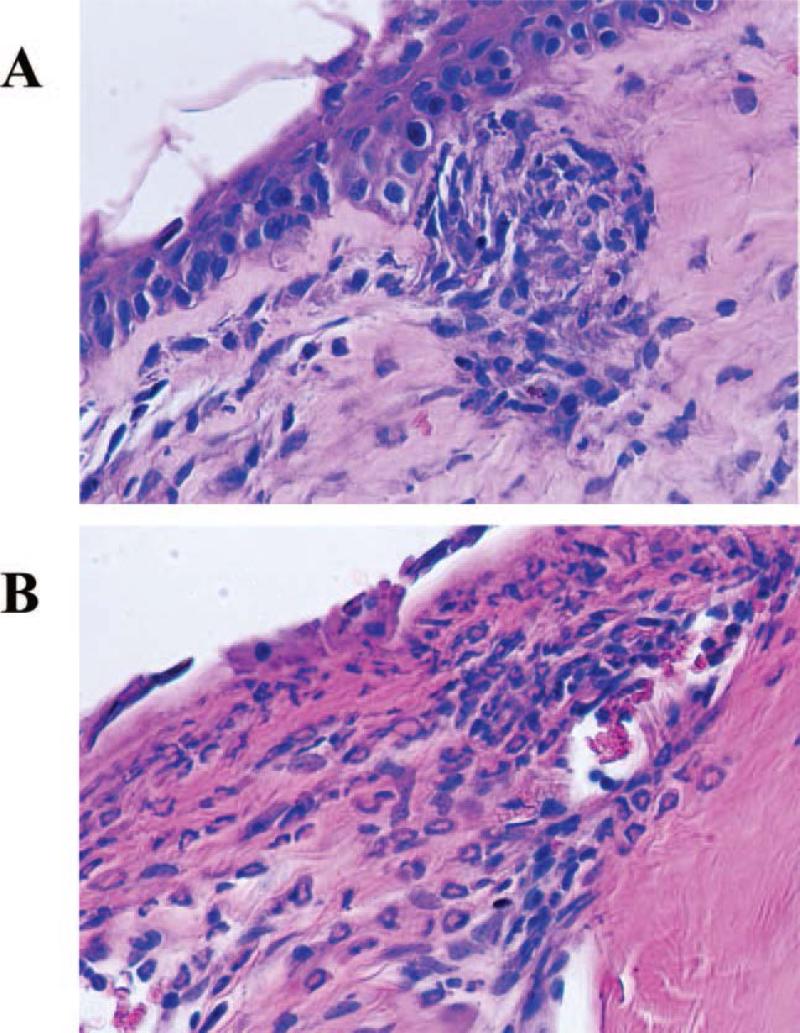
Figure 8.
Difference in infiltrating inflammatory cell types. Penetrating grafts showed mild inflammatory cell infiltration in the stroma consisting primarily of mononuclear cells (PK graft undergoing rejection on day 10, (A)). In contrast, the infiltrate in LK grafts consisted of more polymorphonuclear cells (neutrophils) than mononuclear cells (LK graft undergoing rejection on day 15 (B)).
DISCUSSION
We established a novel model of LK in the mouse and used this model to compare the clinical course and histological features with the conventional PK model for allogeneic transplantation. In this study, syngeneic transplant controls were not performed alongside allogeneic PK and LK surgeries, but controls that address the effect of the surgical technique were included, and technical (surgical) failures were omitted from analysis. Our results in this preliminary study directly comparing surgical outcomes of PK vs. LK in the mouse showed that 69.2% of lamellar grafts were rejected after allogeneic transplantation, compared to rejection of 100% of penetrating corneal grafts using a similar murine strain combination. We found the mean survival time (MST) to be 7.7 days for the PK group, while other investigators have reported survival of PKs of the same strain combination for several weeks22,23. Although we are not certain of the reason for this difference, the low MST in this study could be related to the removal of sutures on day 7. The removal of sutures was performed in both PK and LK groups, so the MST of LKs could also potentially be higher without this removal step.
Furthermore, some rejected lamellar grafts (2/9 rejected grafts) eventually cleared, but none of the rejected penetrating grafts became clear again. Not only was the rate of neovascularization lower in LK grafts compared to PK grafts (84.6% vs. 100%), but severity of neovascularization in LK was also different (Fig. 3C). In all cases neovascularization was observed at the same time rejection occurred, which strongly suggested that neovascularization was correlated with rejection, regardless of whether the surgical procedure was LK or PK. Our results support the hypothesis that the donor endothelium plays a major role in immunological rejection of the transplanted cornea since LK and PK differ essentially only in this regard.
Consistent with the clinical findings, histological observation revealed distinguishing characteristics between LK and PK. Mixed cellular infiltration consisting of mononuclear cells (probably lymphocytes), cells with dendritic morphology, and polymorphonuclear leukocytes (neutrophils) were observed in both LK and PK when rejection occurred. Approximately twice as many neutrophils were seen in LK compared to PK (60% vs. 30%).
Corneal graft rejection in murine PK models has been characterized by a mononuclear inflammatory infiltrate and Th1 cells have been reported to be pivotal24. Our findings of a different inflammatory cell composition and distinct clinical observations in LK compared to PK during rejection suggest different immune mechanisms at work. This model would be useful for further study of distinguishing immunological pathways mediating the inflammatory response and graft rejection, e.g., role of T cell sensitization.
To date, all experimental models of corneal transplantation have been of penetrating keratoplasty. To our knowledge, the only exception is a murine model of interlamellar corneal transplantation, established by Lau et al.18, who developed this model to avoid some drawbacks of the PK model. They noted that histologically, mild cellular infiltration and neovascularization were seen only around the donor endothelium, even though no clinical rejection was noted. Differing results with Lau et al. might be due in part to the different surgical techniques as well as different strain combinations and MHC disparities.
The lamellar graft microenvironment might support the persistence and proliferation of the epithelial cyst between donor button and recipient bed. Since donor epithelium has been found to be a major target of rejection25,26, this interlamellar epithelial proliferation could induce rejection. We noted that epithelial cysts were usually accompanied by minimal infiltration and neovascularization, although no indicator of clinical rejection was present. Since epithelial proliferation only occurred in LK, not in PK, it is a distinct feature of corneal graft response in LK vs. PK.
This preliminary study confirms the feasibility of performing lamellar keratoplasty in the mouse and it is the first study to our knowledge to compare LK with PK. Graft rejection appears to differ in LK compared to PK, both clinically and histologically. This murine LK model will be useful for future investigations into specific immunological mechanisms in lamellar vs. penetrating grafts.
ACKNOWLEDGEMENTS
This work was supported by a grant from the International Cooperation Program of Guangdong Province, grants EY06484 and EY13093 from the NIH, grants from Research to Prevent Blindness, and a grant from the Rosenfeld Family Trust.
The authors also wish to thank Friederike Mackensen, Robert Gould, Michael Davies, and Timothy Chipps for technical assistance.
REFERENCES
- 1.Terry MA. The evolution of lamellar grafting techniques over twenty-five years. Cornea. 2000;19:611–616. doi: 10.1097/00003226-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 2.The Collaborative Corneal Transplantation Studies Research Group The collaborative corneal transplantation studies (CCTS): Effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392–1403. [PubMed] [Google Scholar]
- 3.Cursiefen C, Chen L, Dana MR, et al. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Cursiefen C, Rummelt C, Junemann A, et al. Absence of blood and lymphatic vessels in the developing human cornea. Cornea. 2006;25:722–726. doi: 10.1097/01.ico.0000214230.21238.3d. [DOI] [PubMed] [Google Scholar]
- 5.Niederkorn JY. The immune privilege of corneal grafts. J Leukoc Biol. 2003;74:167–171. doi: 10.1189/jlb.1102543. [DOI] [PubMed] [Google Scholar]
- 6.Wang HM, Kaplan HJ, Chan WC, et al. The distribution and ontogeny of MHC antigens in murine ocular tissue. Invest Ophthalmol Vis Sci. 1987;28:1383–1389. [PubMed] [Google Scholar]
- 7.Tripathi BJ, Tripathi RC, Wong P, et al. Expression of HLA by the human trabecular meshwork and corneal endothelium. Exp Eye Res. 1990;51:269–276. doi: 10.1016/0014-4835(90)90023-n. [DOI] [PubMed] [Google Scholar]
- 8.Abi-Hanna D, Wakefield D, Watkins S. HLA antigens in ocular tissues. I. In vivo expression in human eyes. Transplantation. 1988;45:610–613. doi: 10.1097/00007890-198803000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Sohn JH, Kaplan HJ, Suk HJ, et al. Complement regulatory activity of normal human intraocular fluid is mediated by MCP, DAF, and CD59. Invest Ophthalmol Vis Sci. 2000;41:4195–4202. [PMC free article] [PubMed] [Google Scholar]
- 10.Sohn JH, Kaplan HJ, Suk HJ, et al. Chronic low level complement activation within the eye is controlled by intraocular complement regulatory proteins. Invest Ophthalmol Vis Sci. 2000;41:3492–3502. [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor AW, Streilein JW, Cousins SW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr Eye Res. 1992;11:1199–1206. doi: 10.3109/02713689208999545. [DOI] [PubMed] [Google Scholar]
- 12.Cousins SW, McCabe MM, Danielpour D, et al. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201–2211. [PubMed] [Google Scholar]
- 13.Stuart PM, Griffith TS, Usui N, et al. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997;99:396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamagami S, Kawashima H, Tsuru T, et al. Role of Fas-Fas ligand interactions in the immunorejection of allogeneic mouse corneal transplants. Transplantation. 1997;64:1107–1111. doi: 10.1097/00007890-199710270-00004. [DOI] [PubMed] [Google Scholar]
- 15.Niederkorn JY. Anterior chamber-associated immune deviation. Chem Immunol. 1999;73:59–71. doi: 10.1159/000058740. [DOI] [PubMed] [Google Scholar]
- 16.Niederkorn JY, Mayhew E, Mellon J, et al. Role of tumor necrosis factor receptor expression in anterior chamber-associated immune deviation (ACAID) and corneal allograft survival. Invest Ophthalmol Vis Sci. 2004;45:2674–2681. doi: 10.1167/iovs.04-0144. [DOI] [PubMed] [Google Scholar]
- 17.Sonoda A, Sonoda Y, Muramatu R, et al. ACAID induced by allogeneic corneal tissue promotes subsequent survival of orthotopic corneal grafts. Invest Ophthalmol Vis Sci. 2000;41:790–798. [PubMed] [Google Scholar]
- 18.Lau CH, Nicholls SM, Easty DL. A murine model of interlamellar corneal transplantation. Br J Ophthalmol. 1998;82:294–9. doi: 10.1136/bjo.82.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khodadoust AA, Silverstein AM. Transplantation and rejection of individual cell layers of the cornea. Invest Ophthalmol. 1969;8:180–195. [PubMed] [Google Scholar]
- 20.Polack FM. Scanning electron microscopy of corneal graft rejection: epithelial rejection, endothelial rejection, and formation of posterior graft membranes. Invest Ophthalmol. 1972;11:1–14. [PubMed] [Google Scholar]
- 21.Stuart PM, Pan F, Yin X, et al. Effect of metalloprotease inhibitors on corneal allograft survival. Invest Ophthalmol Vis Sci. 2004;45:1169–1173. doi: 10.1167/iovs.03-0932. [DOI] [PubMed] [Google Scholar]
- 22.Sonoda Y, Streilein JW. Orthotopic corneal tranplantation in mice - evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54:694–704. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Yamada J, Streilein JW. Fate of orthotopic corneal allografts in C57BL/6 mice. Trans Immunol. 1998;6:161–168. doi: 10.1016/s0966-3274(98)80041-5. [DOI] [PubMed] [Google Scholar]
- 24.Hargrave SL, Hay C, Mellon J, et al. Fate of MHC-matched corneal allografts in Th1-deficient hosts. Invest Ophthalmol Vis Sci. 2004;45:1188–1193. doi: 10.1167/iovs.03-0515. [DOI] [PubMed] [Google Scholar]
- 25.Yao YF, Inoue Y, Miyazaki D, et al. Ocular resurfacing and alloepithelial rejection in a murine keratoepithelioplasty model. Invest Ophthalmol Vis Sci. 1995;36:2623–2633. [PubMed] [Google Scholar]
- 26.Driebe WT, Park JY, Meisler DM. Epithelial rejection rings. Arch Ophthalmol. 1997;115:938–939. doi: 10.1001/archopht.1997.01100160108027. [DOI] [PubMed] [Google Scholar]