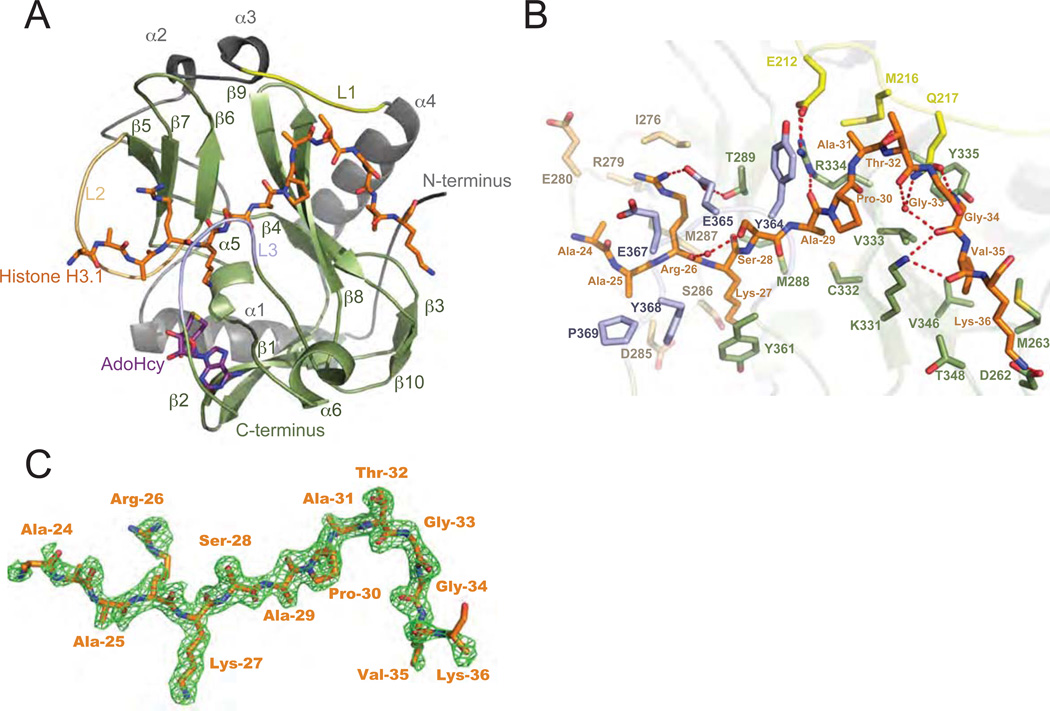
Figure 2. ATXR5/6 contain a bipartite catalytic domain composed of nSET and the SET domain.
A. Ribbons representation of the RcATXR5-H3.1-AdoHcy ternary complex in which nSET and the SET domain are highlighted in grey and green, respectively. Carbon atoms of histone H3.1 and product cofactor are colored in orange and magenta, respectively. L1-L3: loop 1-loop 3. β1–β10: beta strand 1-beta stand 10. α1–α6: alpha helix 1-alpha helix 6. B. Zoomed-view of the peptide binding cleft of RcATXR5. Three-letter code refers to histone H3.1 residues; one-letter code refers to RcATXR5 residues. Carbon atoms of residues found in the L1, L2 and L3 loops are rendered in yellow, beige and purple, respectively. The carbon atoms of other residues interacting with histone H3.1 are highlighted in green. Carbon atoms of histone H3.1 residues are colored in orange while oxygen and nitrogen atoms are highlighted in red and blue. Hydrogen bonds and water molecules are illustrated as red dashed lines and red spheres, respectively. C. Simulated annealing Fo – Fc omit map (green) contoured at 2σ. The histone H3.1 peptide is rendered as in panel A.