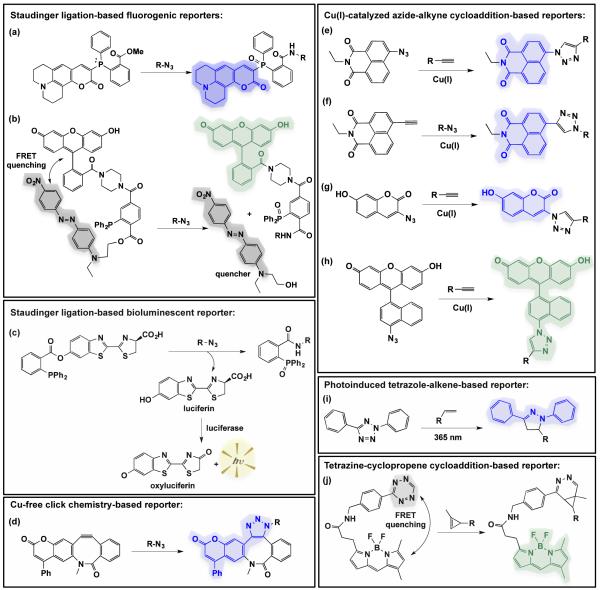
Fig. 7.
Probes that are activated by bioorthogonal chemistries in cell-based settings. (a, b) Staudinger ligation-based fluorogenic reporters are activated by reaction with azides to yield fluorescent products. The probe in (a) is initially quenched by the phosphine lone pair electrons147, whereas the probe in (b) utilizes a FRET quencher that is released upon Staudinger ligation83. (c) Staudinger ligation-based bioluminescent reporter that yields luciferin upon reaction with azides.148 Once luciferin is released, luciferase-expressing cells catalyse the conversion of luciferin to ocyluciferin in a bioluminescent reaction that yields visible light. (d) Cu-free click chemistry-based reporter becomes fluorescent upon reaction with azides.149 (e-h) Several Cu(I)-catalysed azide alkyne cycloaddition-based reporters become fluorescent when the azide or alkyne-based probe reacts with alkynes or azides, respectively.70, 150, 151 (i) Photoinduced tetrazole-based reporter fluoresces blue upon reaction with alkenes.115 (j) Tetrazine quenches BODIPY fluorescence; the conjugate becomes fluorescent when the tetrazine reacts with cyclopropene to yield a cycloadduct.152