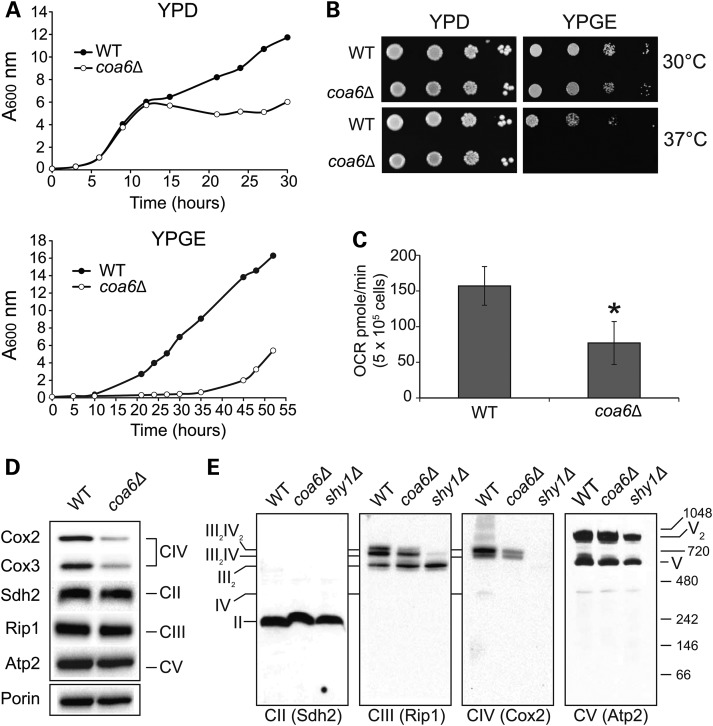
Figure 1.
Yeast coa6Δ cells exhibit reduced respiration and diminished CcO assembly. (A) WT and coa6Δ cells were precultured in YPD and inoculated in fresh YPD or YPGE liquid media at 30°C with a starting A600 of 0.1. Absorbance was measured at the indicated times at 600 nm. Data are representative of at least three independent measurements. (B) Serial dilutions of WT and coa6Δ cells were spotted on YPD and YPGE plates at 30°C and 37°C. Pictures were taken after 2–5 days of spotting. Data are representative of three independent experiments. (C) WT and coa6Δ cells were grown overnight in YPD at 30°C. After 18 h of growth, cells were harvested, washed, counted and resuspended in ethanol-containing respiratory medium. Basal oxygen consumption rate (OCR) was measured on half a million cells using an extracellular flux analyzer. Error bars represent mean ± SD (n = 10), * denotes statistically significant differences, P < 0.001, t-test. (D) Mitochondria were isolated from WT and coa6Δ cells grown to early stationary phase in YPD medium. Mitochondrial protein was extracted and analyzed by SDSPAGE/western blot. Subunit-specific antibodies were used to detect MRC complexes II–V. Porin was used as loading control. (E) Mitochondria from (D) and shy1Δ were solubilized in 1% digitonin, followed by BNPAGE/western blot of native MRC complexes. The shy1Δ cells lack CcO and thus were used as a control for CcO assembly. The stoichiometry and molecular weights of the supercomplexes are indicated.