Abstract
Purpose of review
Ghrelin is a multifaceted gut hormone which activates its receptor, growth hormone secretagogue receptor (GHS-R). Ghrelin's hallmark functions are its stimulatory effects on food intake, fat deposition and growth hormone release. Ghrelin is famously known as the “hunger hormone”. However, ample literature indicates that the functions of ghrelin go well beyond its role as an orexigenic signal. Here we have reviewed some of the most recent findings on ghrelin and its signaling in animals and humans.
Recent findings
Ghrelin regulates glucose hemostasis by inhibiting insulin secretion and regulating gluconeogenesis/glycogenolysis. Ghrelin signaling decreases thermogenesis to regulate energy expenditure. Ghrelin improves the survival prognosis of myocardial infarction by reducing sympathetic nerve activity. Ghrelin prevents muscle atrophy by inducing muscle differentiation and fusion. Ghrelin regulates bone formation and metabolism by modulating proliferation and differentiation of osteoblasts. Ghrelin is also involved in cancer development and metastasis; ghrelin and GHS-R mRNA are highly expressed in metastatic forms of cancers.
Summary
In addition to ghrelin's effects on appetite and adiposity, ghrelin signaling also plays crucial roles in glucose- and energy-homeostasis, cardioprotection, muscle atrophy, bone metabolism and cancer. These multifaceted roles of ghrelin make ghrelin and GHS-R highly attractive targets for drug development.
Keywords: ghrelin, GHS-R, glucose- and energy- homeostasis, heart and muscle, bone and cancer
Introduction
Ghrelin is a 28-aa Ser3 acylated peptide, and its functionally relevant endogenous receptor is growth hormone secretagogue receptor (GHS-R). Ghrelin is a key regulator of nutrient sensing, meal initiation, and appetite (1, 2). Apart from its orexigenic effect, research in the last decade has shown that ghrelin has regulatory roles in in many organs and systems (1–3). Ghrelin signaling has increasingly been recognized as a key regulator of obesity, insulin resistance and diabetes; intriguingly, many of these regulatory functions appear to be independent of ghrelin's effect on food intake (4–8). This current review is focused on the most recent findings of ghrelin in glucose homeostasis (9–11), energy-homeostasis (7, 12), heart disease (13–16), muscular atrophy (17, 18), bone metabolism (8, 19, 20), and cancer development/progression (21, 22).
1) Glucose homeostasis
Glucose homeostasis is a highly complex process, controlled by neuroendocrine hormones that regulate glucose uptake, storage and disposal processes. Ghrelin has been shown to play an important role in glucose homeostasis. The majority of studies report that ghrelin inhibits insulin secretion; although some studies also observe stimulation or no effect on insulin secretion (23). The differential effects of ghrelin on insulin secretion appear to be correlated with ghrelin and/or glycemic conditions (23). A recent study suggests that ghrelin's effect on insulin secretion depends on the heteromerization between GHS-R and the somatostatin receptor 5, both of which are G-protein-coupled receptors (GPCRs) (24). The heteromerization varies according to the ratio of somatostatin:ghrelin in the serum; this ratio determines whether the Gα(i/o) subunit or the Gα(q) subunit is being activated, which leads to activation of different signal transduction pathways that result in different insulin responses to ghrelin (24).
Studies using leptin-deficient obese and diabetic ob/ob mice and ghrelin−/−.ob/ob mice demonstrated that the ablation of ghrelin improves the diabetic phenotype with increased insulin secretion, reduced blood glucose and improved glucose tolerance (6). Mitochondrial uncoupling protein 2 (UCP2) decreases ATP production to lower the ATP:ADP ratio, a key signal needed for insulin secretion (25). The ghrelin−/−.ob/ob mice have lower UCP2 expression than that of ob/ob mice, suggesting that ghrelin negatively affects insulin secretion by regulating the expression of UCP2 in the pancreatic β-cells (6). Surprisingly, studies involving ghrelin receptor knockout mice (Ghsr−/−) suggest that GHS-R has an opposite effect on glucose homeostasis compared to ghrelin (9). The hyperglycemic phenotype of Ghsr−/−:ob/ob mice is actually worse than that of ob/ob mice. The Ghsr−/−:ob/ob mice have lower insulin, higher blood glucose, and worsened glucose tolerance (9). Histological analysis of islets reveals that Ghsr−/−:ob/ob mice have islets similar in size compared to ob/ob mice, which implies that the β-cell mass of the islets is not affected in the mice; this points toward an effect of β-cell function. Unlike ghrelin−/−:ob/ob mice, UCP2 expression is significantly higher in Ghsr−/−:ob/ob mice, explaining the reduced insulin secretion (9). Given the paradoxical findings of ghrelin ablation and GHS-R ablation on glycemic control, ghrelin's inhibitory effect on insulin secretion may be mediated by a yet unknown receptor(s) other than GHS-R. These results highlight the complexity of the ghrelin-signaling pathway in pancreatic β-cells; thus it is critically important to distinguish the effects of ghrelin antagonism from that of GHS-R antagonism for the regulation of glucose homeostasis.
Ghrelin also has been shown to be essential in maintaining glucose homeostasis during starvation. Ghrelin acylation is activated by ghrelin O-acyltransferase (GOAT). GOAT−/− mice exhibit extreme hypoglycemia under 60% calorie restriction (26). The hypoglycemic phenotype of GOAT−/− mice can be rescued by infusion of ghrelin, growth hormone (GH), lactate or fatty acid (10). This suggests that ghrelin increases glucose production by activating gluconeogenic processes and/or growth hormone-regulated pathways. However, a recent study of GOAT−/−, ghrelin−/−, Ghsr−/− andghrelin−/− and Ghsr−/− double knockout mice under 50% calorie restriction failed to observe this extreme hypoglycemia (27).
The counter-regulatory response to hypoglycemia is an important component of glucose homeostasis. This response is mediated by glucagon from the pancreatic islets (28, 29), catecholamines from adrenal glands (30), and growth hormone (GH) from the anterior pituitary (31), which synergistically increase hepatic glucose output. There is evidence of feedback regulation between ghrelin and glucagon. A study in mouse islets and α-cells demonstrates that ghrelin, via GHS-R, directly stimulates glucagon secretion in pancreatic α-cells (32). Ghrelin's effect on glucagon secretion is regulated by intracellular calcium flux and phosphorylation of extracellular-signal-regulated kinase (ERK) (32). Conversely, glucagon, along with norepinephrine, has been shown to stimulate ghrelin secretion via the MAPK and EPAC pathways in nutrient-deficient conditions (33).
Glucose production by the liver is an important therapeutic target for the treatment of type 2 diabetes (T2D). Metformin, a drug commonly used to treat T2DM, reduces hepatic gluconeogenesis (34). In primary gastric cells, it is demonstrated that metformin treatment inhibits ghrelin production through activation of AMP-activated protein kinase (AMPK) (35). This portends an interesting connection between the anti-diabetic effects of metformin and ghrelin's inhibitory effect on insulin secretion. Studies using ghrelin−/− and Ghsr−/− mice recently reveal increased gluconeogenesis and glycogenolysis in hepatic tissues during the post-absorptive state, suggesting that the ghrelin-GHS-R axis may inhibit the production of glucose and the breakdown of glycogen in the liver (11). It is worth noting that this study was done in a post-absorptive state; it may not reflect glucose regulation under fasting condition when glucagon is activated.
The central nervous system (CNS) also has an important role in the regulation of glucose homeostasis. In a recent study, a Phox2b-Cre-driven system was utilized to induce GHS-R expression exclusively in the hindbrain region of global GHS-R knockout mice (36). Global GHS-R KO mice exhibit hypoglycemia under fasting conditions, but re-expression of GHS-R in the hindbrain only rescues the hypoglycemic phenotype but not ghrelin-induced food intake (36). Notably, systemic ghrelin injections increase the cFos expression in the hindbrain of wild-type mice, but not in the Phox2b-Cre-driven GHSR re-expression model; this suggests that GHS-R action in hindbrain is independent of ghrelin (36). In another report, ghrelin stimulates the dopaminergic neurons in the ventral tegmental area (VTA), but re-expression of GHS-R in these neurons cannot rescue fasting hypoglycemia, although it partially increases food intake (37). These data suggest the glycemic and orexigenic effects of ghrelin signaling are regulated at different neurons in the brain.
2) Energy homeostasis
Energy homeostasis depends on the balance between energy intake and energy expenditure. Dysregulation of energy homeostasis results in obesity or anorexia. It is well documented that ghrelin is orexigenic, increasing energy intake (1–3, 38, 39). Central and peripheral administration of ghrelin increases adipogenesis and lipogenesis, but decreases lipolysis. Interestingly, the adipogenic effects appear to be independent from ghrelin's orexigenic function (4, 5).
Along with energy intake, ghrelin also affects energy expenditure. It was recently reported that administration of ghrelin antibody to mice increases fasting energy expenditure (12). This study suggests that ghrelin can regulate energy balance in addition to energy intake. Adult Ghsr−/− mice show reduced body weight (40); the phenotype becomes more pronounced in older (9 month or older) Ghsr−/− mice. Older Ghsr−/− mice are lean and insulin-sensitive, but no difference in food intake or physical activity (7). Consistently, the older Ghsr−/− mice show a healthier lipid profile with decreased cholesterol and triglycerides, and improved insulin sensitivity. The metabolic profile analyses indicate that the lean and insulin-sensitive phenotype of older Ghsr−/− mice is due to increased energy expenditure (7). Recent studies have shown that non-shivering thermogenesis in brown fat plays a crucial role in energy balance in adult humans; reduced non-shivering thermogenesis leads to decreasing energy expenditure and obesity (41–43). Older Ghsr−/− mice have a higher core body temperature due to increased thermogenesis in brown fat (7). Further cellular and molecular characterization reveals that there is higher mitochondrial content and elevated expression of uncoupling protein 1 (UCP1) in the brown fat (7). In the presence of fatty acids, UCP1 (also called thermogenin) uncouples oxidative phosphorylation from ATP synthase by facilitating proton movement across the mitochondrial inner membrane, resulting in heat generation (41). The thermogenic phenotype of Ghsr−/− mice indicates that GHS-R is an important regulator of thermogenesis. GHS-R ablation increases energy expenditure independent of food intake or physical activity, suggesting that GHS-R antagonists may represent a novel approach to combat obesity without the need for dieting or exercise.
3) Cardiac functions
Ghrelin is present in human cardiomyocytes. Myocardial infarction (MI) often is accompanied by malignant ventricular arrhythmia and increased cardiac sympathetic nerve activity (CSNA) (44). Ghrelin has been shown to act on the vagal nerves to prevent the increase of CSNA and arrhythmia, improving the survival prognosis of MI (13). Furthermore, during the recovery phase after MI, ghrelin prevents excessive sympathetic activation by reducing epinephrine and norepinephrine, thus improving the function of the heart (14).
In rodent models, ghrelin administration induces changes which promote angiogenesis. A recent study in a rat model of cardiopulmonary bypass (CPB) shows that ghrelin treatment reduces inflammatory responses, apoptosis and oxidative stress induced by CPB, and preserves the cardiac pumping function through GHS-R1a and Akt signaling (15). Increased angiogenesis is measured by changes in vascular endothelial growth factor (VEGF), VEGF receptor, and nitric oxide (NO) (45). Ghrelin treatment in rat model of MI demonstrates increased VEGF expression and angiogenesis (16). In diet-induced obese mice, systemic ghrelin treatment increases serum VEGF, and decreases NO concentrations (45). Overall, ghrelin treatment may reduce CSNA inflammation and oxidative stress in the heart, and induce angiogenesis. More studies are needed to further assess whether ghrelin is beneficial for treating heart diseases.
4) Muscle atrophy
Muscle atrophy, characterized by reduced muscle mass and impaired muscle function, accompanies many diseases, including cachexia from cancer or AIDS, and muscle denervation. Ghrelin can indirectly increase muscle mass by increasing food intake and activating the GH/Insulin-like growth factor-1 (IGF-1) axis in cachexic mice (17). There is also evidence of direct cellular effects. It has been shown that ghrelin promotes myocyte differentiation and fusion in C2C12 myoblasts (46). A recent study further demonstrates that the protective effect of ghrelin in fasting- and denervation-induced muscle atrophy is mediated by mTOR and Akt signaling (18). Intriguingly, this study shows that both acylated and unacylated ghrelin have protective effects on muscle atrophy (18). Of note, these anti-atrophic effects do not appear to be associated with the activation of the GH/IGF-1 axis. Also since unacylated ghrelin does not bind to GHS-R, the muscular functions of ghrelin and unacylated ghrelin are likely to be mediated by a yet-unidentified receptor (18).
5) Bone metabolism
Ghrelin and GHS-R1a are expressed in rat osteoblasts; ghrelin increases proliferation and differentiation of osteoblasts in vitro and bone mineral density (BMD) in vivo (47). It has been shown that ghrelin regulates bone formation and mass by activating phosphorylation of AMPK (48). Ghrelin treatment has no effect on differentiation of rat osteoclasts, but promotes proliferation of human osteoblasts, although these cells only express the inactive receptor isoform GHS-R1b (49). The result suggests that ghrelin's effect on bone turnover may not be mediated by GHS-R1a. It is recently reported that ghrelin, interacting with leptin, regulates osteoclastogenesis and bone metabolism in an age-dependent manner (19, 20). Ghrelin has dual roles in osteoclastogenesis: inhibiting osteoclast progenitors locally, but stimulating osteoclastogenesis systemically (19). Ghrelin and leptin have opposite effects on bone metabolism; ghrelin's systemic osteoclastogenic activity is suppressed by leptin (19). Intriguingly, ghrelin's systemic osteoclastogenic effect diminishes with age, which unmasks its direct protective effect on bone formation (19). This study has significant therapeutic implications, suggesting that elderly osteoporosis patients may benefit from ghrelin therapy. Most recently, a study in rats shows that chronic central administration of ghrelin increases bone mass independent of food intake or weight gain (8). A study in healthy elderly women shows positive correlation between serum ghrelin and trabecular BMD, using quantitative CT (50). Thus in general, ghrelin promotes bone formation and increases bone mass; however, its effect on bone metabolism is age-dependent.
6) Cancer development and progression
Ghrelin and GHS-R have been detected in many endocrine and non-endocrine tumors (21, 22), suggesting that the ghrelin/GHS-R axis might be associated with tumor growth and progression. In pituitary tumors, ghrelin mRNA is detected in non-functional adenomas, GH- and gonadotropin-producing adenomas and prolactinomas, with highest GHS-R expression detected in the GH-producing adenomas (51). A variant of human preproghrelin (In1-ghrelin), which has Exons 0–1, intron 1, and Exon 2 and lacks Exons 3–4 is detected in human breast cancer tissue (52). The expression levels of In1-ghrelin increases 8-fold in ductal breast cancer samples compared to normal breast tissues, and strongly correlates with GOAT expression, suggesting that In1-ghrelin might be a substrate for GOAT acylation. Intriguingly, GOAT and GHS-R1b expression is significantly higher in breast cancer tissues compared to normal tissues (52). Tamoxifen is currently used as primary therapy for estrogen receptor-positive breast cancers. Interestingly, tamoxifen increases native ghrelin expression, but reduces In1-ghrelin expression (52).
Various studies have reported that ghrelin exhibits proliferative properties in cancer (21). A recent study in canine mammary carcinoma shows high levels of ghrelin and GHS-R in metastatic tumors (22). Low doses of ghrelin stimulate cellular proliferation, inhibit apoptosis, and promote motility and invasion by canine mammary cancer cells. Significantly, blockade of ghrelin's receptor, GHS-R, enhances early apoptosis (22). These studies are intriguing, which foster the hypothesis that ghrelin may promote cancer growth and metastasis, and GHS-R antagonist has anti-cancer properties.
Conclusions
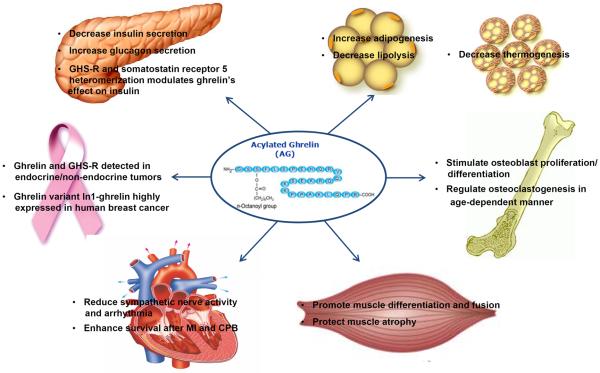
In addition to its role as the “hunger hormone”, ghrelin has much broader functions (Figure 1). Ghrelin regulates glucose homeostasis through inhibition of insulin secretion and regulation of hepatic glucose output. Ghrelin signaling regulates energy homeostasis by decreasing thermogenesis to reduce energy expenditure. Ghrelin also has cardio-protective effects in the myocardium and anti-atrophic effects in muscle. Moreover, ghrelin has a role in bone formation/metabolism and may also be a potential target for cancer. All of these roles reveal the exciting therapeutic potential for targeting ghrelin and/or its receptor. However, since ghrelin and GHS-R have effects on many organs with both beneficial and detrimental effects, caution must be taken to avoid side effects when developing therapies.
Figure 1.
Summary of non-orexigenic functions of ghrelin and its signaling.
Key Points.
-
1)
Ghrelin ablation improves glucose homeostasis, whereas GHS-R ablation worsens it.
-
2)
GHS-R ablation increases energy expenditure, attenuating age-associated obesity and insulin resistance.
-
3)
Ghrelin reduces sympathetic nerve activity, and has a cardio-protective effect.
-
4)
Ghrelin prevents muscle atrophy.
-
5)
Ghrelin regulates bone formation/bone metabolism, and may promote cancer development and metastasis.
Acknowledgements
This article was supported by the USDA/ARS under Cooperative Agreement No. 58-6250-0-008 (YS), the American Heart Association (AHA) innovative grant 12IRG9230004 (YS), and the NIH-Diabetes and Endocrinology Research Center grant P30DK079638 (YS, SLS), the American Diabetes Association and the Baylor College of Medicine Alkek Bridge fund (SLS).
References and recommended reading
Papers of particular interest have been highlighted as:
* of special interest
** of outstanding interest
- 1.Sato T, Nakamura Y, Shiimura Y, Ohgusu H, Kangawa K, Kojima M. Structure, regulation and function of ghrelin. Journal of biochemistry. 2012;151:119–28. doi: 10.1093/jb/mvr134. [DOI] [PubMed] [Google Scholar]
- 2.Kirchner H, Heppner KM, Tschop MH. The role of ghrelin in the control of energy balance. Handbook of experimental pharmacology. 2012:161–84. doi: 10.1007/978-3-642-24716-3_7. [DOI] [PubMed] [Google Scholar]
- 3.Varela L, Vazquez MJ, Cordido F, Nogueiras R, Vidal-Puig A, Dieguez C, et al. Ghrelin and lipid metabolism: key partners in energy balance. Journal of molecular endocrinology. 2011;46:R43–63. doi: 10.1677/JME-10-0068. [DOI] [PubMed] [Google Scholar]
- 4.Andrews ZB, Erion DM, Beiler R, Choi CS, Shulman GI, Horvath TL. Uncoupling Protein-2 Decreases the Lipogenic Actions of Ghrelin. Endocrinology. 2010;151:2078–86. doi: 10.1210/en.2009-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Tilve D, Heppner K, Kirchner H, et al. Ghrelin-induced adiposity is independent of orexigenic effects. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:2814–22. doi: 10.1096/fj.11-183632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Asnicar M, Saha PK, et al. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell metabolism. 2006;3:379–86. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Lin L, Saha PK, Ma X, et al. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell. 2011;10:996–1010. doi: 10.1111/j.1474-9726.2011.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study demonstrates that GHS-R can regulate energy expenditure independent from food intake or physical activity, and reveals for the first time that GHS-R is an important regulator of thermogenesis during aging.
- 8.Choi HJ, Ki KH, Yang JY, Jang BY, Song JA, Baek WY, et al. Chronic Central Administration of Ghrelin Increases Bone Mass through a Mechanism Independent of Appetite Regulation. PLoS One. 2013;8:e65505. doi: 10.1371/journal.pone.0065505. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study in rats shows that chronic ICV administration of ghrelin increases bone mass, and demonstrates the effect is independent from ghrelin's orexigenic function.
- 9.Ma X, Lin Y, Lin L, et al. Ablation of ghrelin receptor in leptin-deficient ob/ob mice has paradoxical effects on glucose homeostasis when compared with ablation of ghrelin in ob/ob mice. Am J Physiol Endocrinol Metab. 2012;303:E422–31. doi: 10.1152/ajpendo.00576.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper shows that deletion of ghrelin or GHS-R in leptin-deficient background results in differential effects on glucose regulation, suggesting that ghrelin's effect on insulin may be independent from GHS-R.
- 10.Li RL, Sherbet DP, Elsbernd BL, et al. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. The Journal of biological chemistry. 2012;287:17942–50. doi: 10.1074/jbc.M112.358051. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This report shows that calorie-restricted and fat-depleted ghrelin-deficient mice exhibit extreme hypoglycemia after overnight fasting, and the phenotype can be rescued by gluconeogenic precursors.
- 11.Chacko SK, Haymond MW, Sun Y, et al. Effect of ghrelin on glucose regulation in mice. Am J Physiol Endocrinol Metab. 2012;302:E1055–62. doi: 10.1152/ajpendo.00445.2011. [DOI] [PubMed] [Google Scholar]; * This paper uses a state-of-the art stable isotope approach to demonstrate that ghrelin signaling regulates gluconeogenesis and glycogenolysis in the liver.
- 12.Zakhari JS, Zorrilla EP, Zhou B, et al. Oligoclonal antibody targeting ghrelin increases energy expenditure and reduces food intake in fasted mice. Molecular pharmaceutics. 2012;9:281–9. doi: 10.1021/mp200376c. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study demonstrates that immunological blocking of ghrelin in the periphery increases energy expenditure, yielding higher heat production. This study is significant, because the approach can be more easily adapted to the clinic.
- 13.Mao Y, Tokudome T, Otani K, Kishimoto I, Nakanishi M, Hosoda H, et al. Ghrelin prevents incidence of malignant arrhythmia after acute myocardial infarction through vagal afferent nerves. Endocrinology. 2012;153:3426–34. doi: 10.1210/en.2012-1065. [DOI] [PubMed] [Google Scholar]; ** This study uses a ghrelin knockout mouse model to demonstrate that the ghrelin gene plays a critical role in cardiac function. More excitingly, it shows that ghrelin treatment of the knockout mice prevents excessive sympathetic activation, decreases plasma catecholamines, and improves heart function and survival after acute myocardial infarction.
- 14.Mao Y, Tokudome T, Otani K, et al. Excessive sympathoactivation and deteriorated heart function after myocardial infarction in male ghrelin knockout mice. Endocrinology. 2013;154:1854–63. doi: 10.1210/en.2012-2132. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Tang J, Yang T, et al. Cardioprotective effect of ghrelin in cardiopulmonary bypass involves a reduction in inflammatory response. PLoS One. 2013;8:e55021. doi: 10.1371/journal.pone.0055021. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper demonstrates that ghrelin treatment not only reduces inflammatory cytokines, apoptosis, oxidative stress and myocardial injury markers, but also improves cardiac function following cardiopulmonary bypass.
- 16.Yuan MJ, He H, Hu HY, et al. Myocardial angiogenesis after chronic ghrelin treatment in a rat myocardial infarction model. Regulatory peptides. 2012;179:39–42. doi: 10.1016/j.regpep.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama M, Yamaki A, Furuya M, et al. Ghrelin improves body weight loss and skeletal muscle catabolism associated with angiotensin II-induced cachexia in mice. Regulatory peptides. 2012;178:21–8. doi: 10.1016/j.regpep.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Porporato PE, Filigheddu N, Reano S, et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. The Journal of clinical investigation. 2013;123:611–22. doi: 10.1172/JCI39920. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is the first in vivo study showing that both ghrelin and unacylated ghrelin have anti-atrophic effects on the skeletal muscle, and the effects are mediated by PI3Kβ-, mTORC2 and p38-mediated pathways. This study also indicates that the protective effect of ghrelin is independent from GHS-R.
- 19.van der Velde M, van der Eerden BC, Sun Y, et al. An age-dependent interaction with leptin unmasks ghrelin's bone-protective effects. Endocrinology. 2012;153:3593–602. doi: 10.1210/en.2012-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Using ghrelin receptor- and leptin-deficient mice, this paper demonstrates that there is an interplay between ghrelin and leptin in the regulation of bone metabolism, and ghrelin and leptin exhibit opposite effects. This is the first study showing that ghrelin's effect on bone is age-dependent.
- 20.McLarnon A. Metabolism: age-dependent balance of leptin and ghrelin regulates bone metabolism. Nature reviews Endocrinology. 2012;8:504. doi: 10.1038/nrendo.2012.116. [DOI] [PubMed] [Google Scholar]
- 21.Majchrzak K, Szyszko K, Pawlowski KM, et al. A role of ghrelin in cancerogenesis. Polish journal of veterinary sciences. 2012;15:189–97. doi: 10.2478/v10181-011-0133-5. [DOI] [PubMed] [Google Scholar]; * This paper shows that ghrelin exhibits proliferative properties in cancer.
- 22.Majchrzak K, Pawlowski KM, Orzechowska EJ, et al. A role of ghrelin in canine mammary carcinoma cells proliferation, apoptosis and migration. BMC Vet Res. 2012;8:170. doi: 10.1186/1746-6148-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper shows that ghrelin and GHS-R are expressed in metastatic tumors. Low doses of ghrelin stimulate cellular proliferation, inhibit apoptosis and promote motility and invasion of canine mammary cancer cells. It was also observed that GHS-R antagonist has anti-cancer effect promoting early apoptosis.
- 23.Verhulst PJ, Depoortere I. Ghrelin's second life: from appetite stimulator to glucose regulator. World J Gastroenterol. 2012;18:3183–95. doi: 10.3748/wjg.v18.i25.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, Jiang H, Zhang H, Smith RG. Modification of ghrelin receptor signaling by somatostatin receptor-5 regulates insulin release. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19003–8. doi: 10.1073/pnas.1209590109. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This article presents a rationale to explain the discrepancy in ghrelin's effect on insulin secretion. It is suggested that differential effects reported in the literature may largely be due to the state of heteromerization between GHS-R1a and somatostatin receptor-5 (SST5). The heteromerization varies depending on the ratio of somatostatin:ghrelin in the serum. This determines which determines whether Gα(i/o) subunit or Gα(q) subunit is being activated, leading to the different responses of ghrelin on insulin secretion.
- 25.Senniappan S, Arya VB, Hussain K. The molecular mechanisms, diagnosis and management of congenital hyperinsulinism. Indian J Endocrinol Metab. 2013;17:19–30. doi: 10.4103/2230-8210.107822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao TJ, Liang G, Li RL, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7467–72. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi CX, Heppner KM, Kirchner H, et al. The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction. PLoS One. 2012;7:e32100. doi: 10.1371/journal.pone.0032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Liang X, Wang S. Intra-islet glucagon secretion and action in the regulation of glucose homeostasis. Frontiers in physiology. 2012;3:485. doi: 10.3389/fphys.2012.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cryer PE. Minireview: Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2012;153:1039–48. doi: 10.1210/en.2011-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. The New England journal of medicine. 2004;350:2272–9. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Hoo RL, Konishi M, et al. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. The Journal of biological chemistry. 2011;286:34559–66. doi: 10.1074/jbc.M111.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuang JC, Sakata I, Kohno D, et al. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol Endocrinol. 2011;25:1600–11. doi: 10.1210/me.2011-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gagnon J, Anini Y. Glucagon stimulates ghrelin secretion through the activation of MAPK and EPAC and potentiates the effect of norepinephrine. Endocrinology. 2013;154:666–74. doi: 10.1210/en.2012-1994. [DOI] [PubMed] [Google Scholar]; * This article shows that glucagon stimulates ghrelin production/secretion in the stomach, via the MAPK and EPAC pathways.
- 34.Viollet B, Guigas B, Sanz Garcia N, et al. Cellular and molecular mechanisms of metformin: an overview. Clinical science. 2012;122:253–70. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gagnon J, Sheppard E, Anini Y. Metformin directly inhibits ghrelin secretion through AMP-activated protein kinase in rat primary gastric cells. Diabetes, obesity & metabolism. 2013;15:276–9. doi: 10.1111/dom.12021. [DOI] [PubMed] [Google Scholar]; * This study shows that the anti-diabetic drug metformin inhibits stomach ghrelin production and secretion by activating AMPK.
- 36.Scott MM, Perello M, Chuang JC, et al. Hindbrain ghrelin receptor signaling is sufficient to maintain fasting glucose. PLoS One. 2012;7:e44089. doi: 10.1371/journal.pone.0044089. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Using a hindbrain-specific GHS-R re-expression mouse model, this article shows that selective re-expression of GHS-R in the hindbrain is not sufficient to restore ghrelin-stimulated food intake, but is sufficient to maintain fasting glucose.
- 37.Chuang JC, Perello M, Sakata I, et al. Ghrelin mediates stress-induced food-reward behavior in mice. The Journal of clinical investigation. 2011;121:2684–92. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 39.Wiedmer P, Nogueiras R, Broglio F, D'Alessio D, Tschop MH. Ghrelin, obesity and diabetes. Nature clinical practice Endocrinology & metabolism. 2007;3:705–12. doi: 10.1038/ncpendmet0625. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proceedings of the National Academy of Sciences. 2004;101:4679–84. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell metabolism. 2010;11:268–72. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Townsend K, Tseng YH. Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential. Adipocyte. 2012;1:13–24. doi: 10.4161/adip.18951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito M. Brown adipose tissue as a regulator of energy expenditure and body fat in humans. Diabetes & metabolism journal. 2013;37:22–9. doi: 10.4093/dmj.2013.37.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jardine DL, Charles CJ, Ashton RK, et al. Increased cardiac sympathetic nerve activity following acute myocardial infarction in a sheep model. The Journal of physiology. 2005;565:325–33. doi: 10.1113/jphysiol.2004.082198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khazaei M, Tahergorabi Z. Systemic ghrelin administration alters serum biomarkers of angiogenesis in diet-induced obese mice. International journal of peptides. 2013;2013:249565. doi: 10.1155/2013/249565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filigheddu N, Gnocchi VF, Coscia M, et al. Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol Biol Cell. 2007;18:986–94. doi: 10.1091/mbc.E06-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delhanty PJ, van der Eerden BC, van Leeuwen JP. Ghrelin and bone. BioFactors. 2013 doi: 10.1002/biof.1120. [DOI] [PubMed] [Google Scholar]
- 48.Shah M, Kola B, Bataveljic A, Arnett TR, Viollet B, Saxon L, et al. AMP-activated protein kinase (AMPK) activation regulates in vitro bone formation and bone mass. Bone. 2010;47:309–19. doi: 10.1016/j.bone.2010.04.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costa JL, Naot D, Lin JM, Watson M, Callon KE, Reid IR, et al. Ghrelin is an Osteoblast Mitogen and Increases Osteoclastic Bone Resorption In Vitro. International journal of peptides. 2011;2011:605193. doi: 10.1155/2011/605193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Napoli N, Pedone C, Pozzilli P, et al. Effect of ghrelin on bone mass density: the InChianti study. Bone. 2011;49:257–63. doi: 10.1016/j.bone.2011.03.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim K, Arai K, Sanno N, et al. Ghrelin and growth hormone (GH) secretagogue receptor (GHSR) mRNA expression in human pituitary adenomas. Clin Endocrinol (Oxf) 2001;54:759–68. doi: 10.1046/j.1365-2265.2001.01286.x. [DOI] [PubMed] [Google Scholar]
- 52.Gahete MD, Cordoba-Chacon J, Hergueta-Redondo M, et al. A novel human ghrelin variant (In1-ghrelin) and ghrelin-O-acyltransferase are overexpressed in breast cancer: potential pathophysiological relevance. PLoS One. 2011;6:e23302. doi: 10.1371/journal.pone.0023302. [DOI] [PMC free article] [PubMed] [Google Scholar]