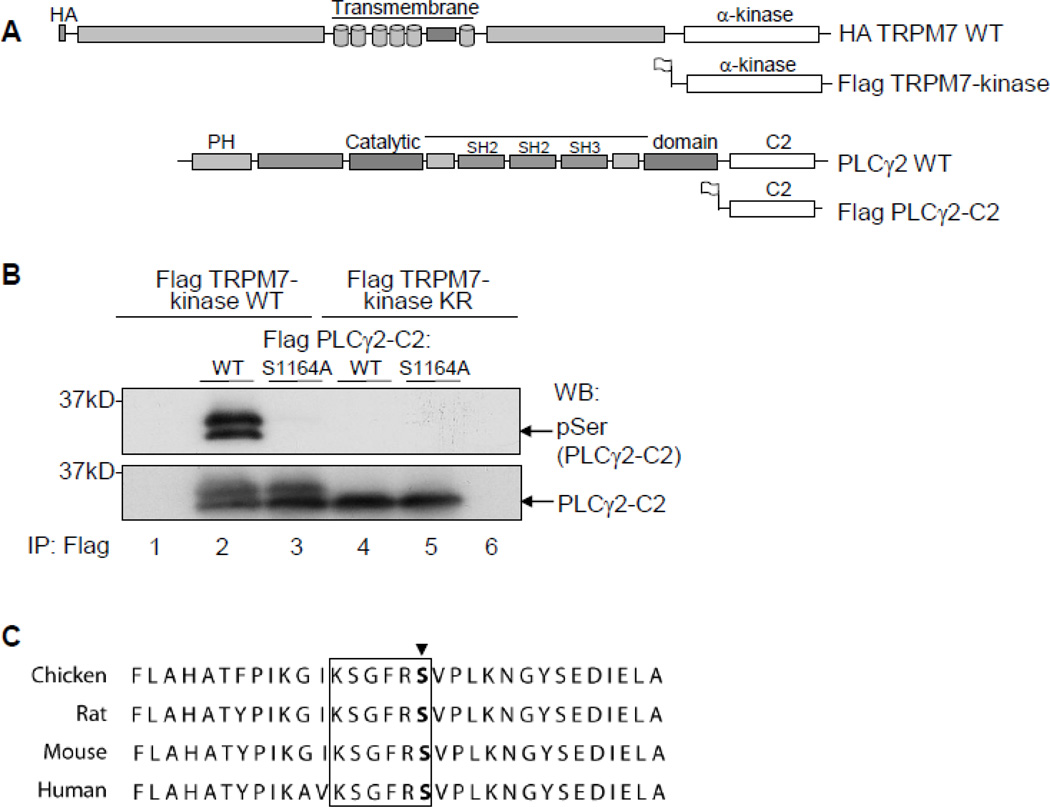
FIGURE 1. The PLCγ2-C2 domain residue, Ser1164, is phosphorylated by TRPM7-kinase.
A. Schematic of the recombinant TRPM7 and PLCγ2 constructs used. B. Phosphorylation of PLCγ2 determined using HEK293 cells transiently transfected with N-terminally Flag-tagged constructs of PLCγ2-C2 WT, PLCγ2-C2 S1164A, TRPM7-kinase WT or TRPM7-kinase dead (KR). Flag dual-immunoprecipitations performed followed by in-vitro phosphorylation reactions. Detection of Ser1164 phosphorylation in PLCγ2 was determined after SDS-PAGE, and immunoblotting with anti-Phospho-Ser motif antibody. C. Alignment of a C-terminal portion of PLCγ2 for chicken, mouse, rat, and human is shown, site Ser1164 marked by arrow, motif specific region is boxed in black.