Figure 3. Quantification of band 3 retention in membrane cytoskeletons following detergent extraction of intact erythrocytes under oxygenated and deoxygenated conditions.
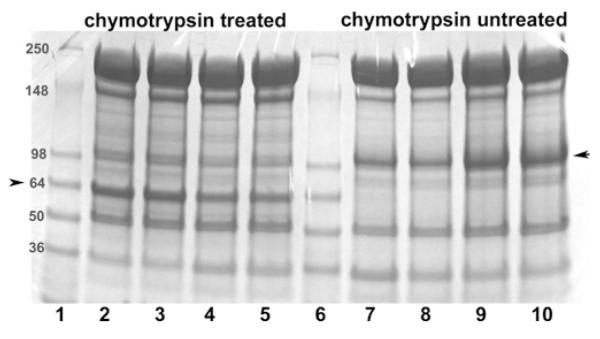
To improve the quantification of band 3 in detergent-extracted membrane cytoskeletons, a fraction of the intact erythrocytes were either treated with chymotrypsin (lanes 2–5) or left unmodified (lanes 7–10). Mild digestion with chymotrypsin selectively cleaves an external loop on band 3, yielding a 65 kDa non-glycosylated N-terminal fragment and 35 kDa glycosylated C-terminal fragment (see the Materials and methods section). Because the non-glycosylated N-terminal fragment migrates more sharply and in an emptier region of the SDS/PAGE gel than intact band 3, it can be more precisely quantified than intact band 3. Importantly, under the digestion conditions used, no other known membrane proteins are cleaved and the two complementary fragments of band 3 remain tightly associated, allowing band 3 to retain all normal functions including unmodified anion transport and ankyrin binding [47,48]. Following the above treatments and prior to detergent extraction, both RBC preparations (digested or undigested) were equilibrated under a gentle stream of humidified argon or air, as described in the Materials and methods section. n-Octyl glucoside (2%) was then added to the sealed tubes and membrane cytoskeletons were extracted by centrifugation through a sucrose cushion at 85000 g. The relative amount of band 3 retained in the spectrin/actin cytoskeletons was then determined using 4–15% gradient SDS/PAGE gels followed by staining with Coomassie Blue. Lanes 1 and 6: molecular mass markers (in kDa); lanes 2 and 3, chymotrypsin-digested oxygenated samples; lanes 4 and 5, chymotrypsin-digested deoxygenated samples; lanes 7 and 8, deoxygenated non-digested samples; and lanes 9 and 10, oxygenated non-digested samples. Each pair of identical lanes was performed on a parallel, but different preparation of erythrocytes and hence constitute independent experiments. The position of band 3 or its 65 kDa fragment is marked with an arrow. The band 3/actin ratio (R) in the cytoskeletal pellet derived from oxygenated and deoxygenated cells was determined using the following formula: R = R oxy/Rdeoxy, where Roxy = band 3oxy/actinoxy and Rdeoxy = band 3deoxy/actindeoxy. Because the presence of residual n-octyl glucoside in the pelleted cytoskeletons, led to somewhat diffuse protein bands, the above experiment was repeated 11 times and the aforementioned ratio was averaged from all eleven experiments, yielding the average value of 1.5 ± 0.17.
