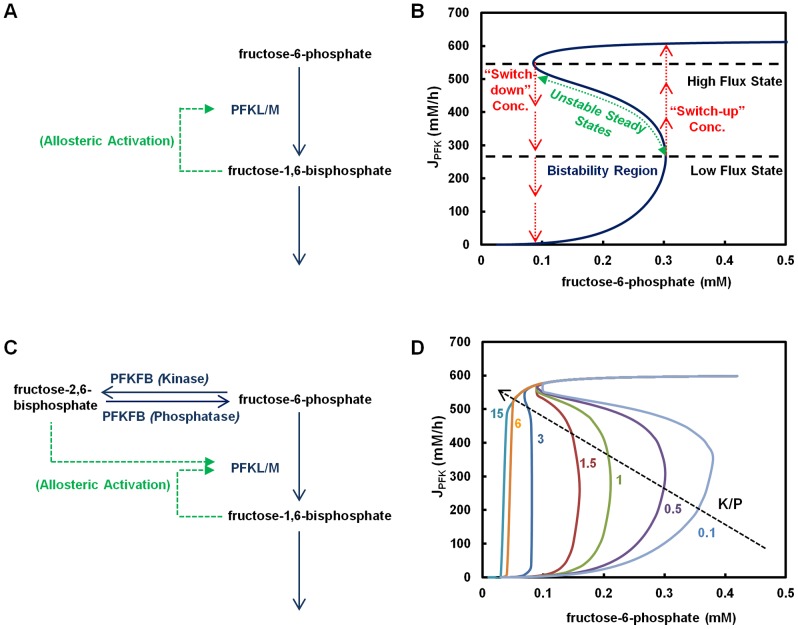
Figure 1. Multiplicity of steady states in kinetics of fructose-6-phosphate node (F6P-node).
(A) Feedback activation of phosphofructokinase (PFK) by fructose-1,6-bisphosphate (F16BP) (B) Bistability in the kinetics of PFK due to feedback regulation by F16BP. The simulation was performed using only two enzymes PFK and aldolase (ALDO). F6P was varied and the steady state PFK flux was solved algebraically. Activation of PFK by F16BP was set at KPFK,fbp = 0.65 mM. (C) Allosteric regulations in the F6P-node. (D) Modulation of bistability span by K/P ratio of PFKFB. The bifunctional enzyme PFKFB was integrated with PFK and ALDO to construct a three enzyme system. Simulations were performed at varying K/P value of PFKFB while keeping all other conditions the same as in (B).