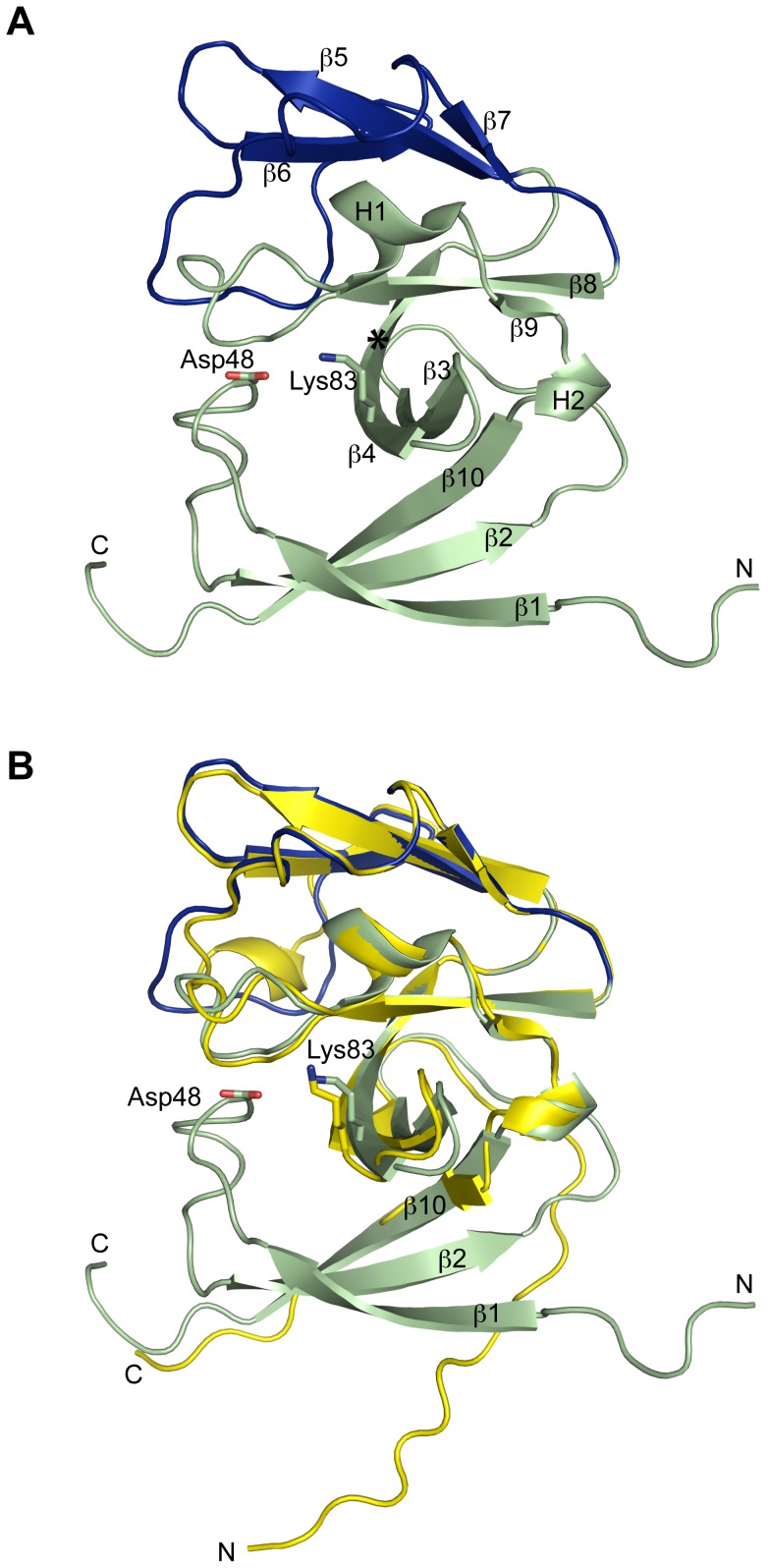
Figure 1. Ribbon diagrams of SipA.
(A) The conserved catalytic core domain is shown in green and the non-catalytic 'cap' domain in blue. β-strands 1, 2 and 10 form a 'membrane associated' anti-parallel β-sheet that is preceded by a transmembrane helix, truncated in this construct. Residues potentially involved in catalytic activity, Asp 48 and Lys 83, are shown in stick form. Residues homologous to SPase-I catalytic dyad are Asp 48 and Gly 85. An asterisk shows the position of Gly 85 (*). (B) Structural superposition of SipA (green/blue) with SipAtrunc (yellow), a previously solved truncated structure, highlights the lack of the 'membrane associated' β-sheet (β-strands 1, 2 and 10) in the truncated structure. Neither the loop that positions Asp48 nor the peptide-binding cleft are present in SipAtrunc. N = N-terminus, C = C-terminus.