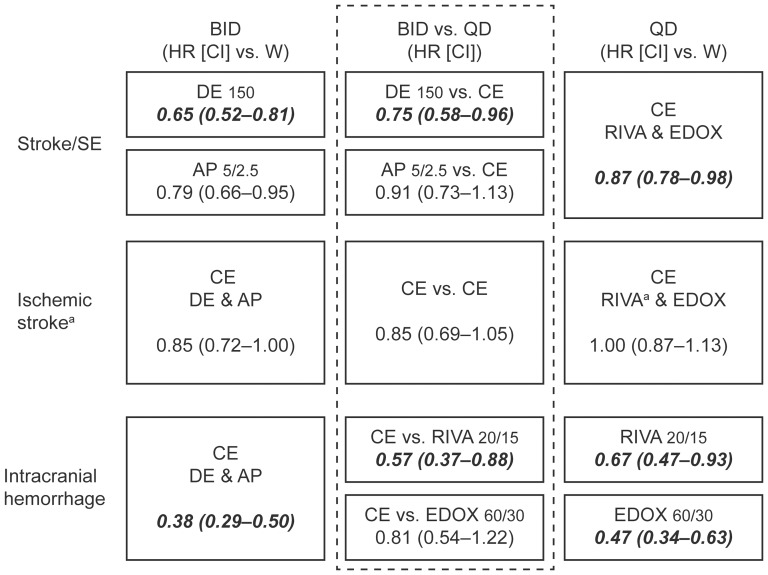
Figure 3. Common estimates where justified and indirect comparisons of all BID or QD dosing regimens of NOACs.
Results are expressed from the respective main dose results of the phase 3 trials [1]–[5] in the intent-to-treat analysis for efficacy (Stroke and systemic embolism, ischemic stroke) and in the safety analysis for intracranial hemorrhage. AP, apixaban; BID, twice-daily dosing; CE, common estimate; CI, confidence interval; DE, dabigatran etexilate; EDOX, edoxaban; HR, hazard ratio; QD, once-daily dosing; RIVA, rivaroxaban; SE, systemic embolism; W, warfarin; aIn the ROCKET-AF trial, only ischemic strokes, excluding unspecified strokes, are reported. Note: bold and italic font marks significantly superior results.