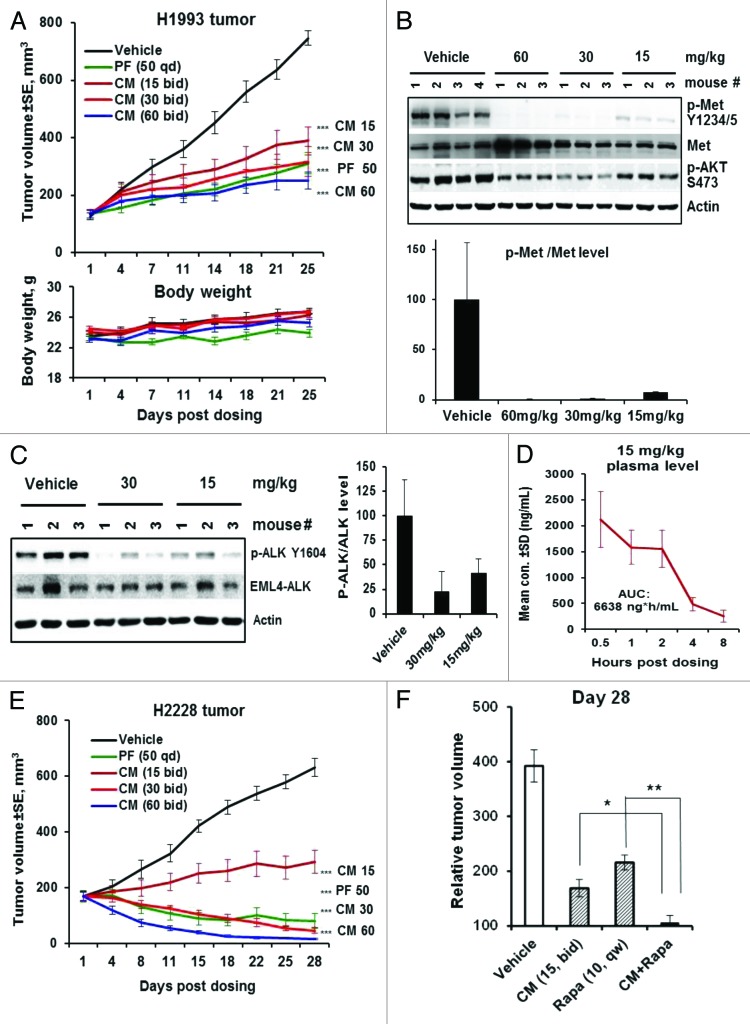
Figure 7. CM-118 shows in vivo efficacy in c-Met-, EML4-ALK-driven NSCLC models. (A) Mice bearing the H1993 tumors (n = 8) were dosed orally with vehicle, 15, 30, 60 mg/kg bid, or 50 mg/kg PF-02341066 (PF) qd for 25 d. Tumor volumes (top) and body weights (bottom) are shown. (B) Tumor lysates made 4 h post the last dose were immunoblotted (top) and quantified (bottom). (C) Mice bearing the H2228 tumors (n = 3) were dosed once with 15 or 30 mg/kg CM-118. Tumor lysates made 4 h post dosing were immunoblotted (left) and quantified (right). (D) Plasma samples (n = 3) of mice received 15 mg/kg CM-118 were analyzed for drug concentration and 8 h exposure AUC. (E) Mice bearing the H2228 tumors (n = 8) were dosed orally with vehicle, 15, 30, 60 mg/kg bid or 50 mg PF qd for 28 d. Tumor volumes are shown. Statistical analysis for (A and E): ***P < 0.001; treated vs. vehicle control. (F) Relative tumor volume (RTV) on day 28 for study groups (n = 8) of vehicle control, 15 mg/kg CM-118 bid, 10 mg/kg rapamycin qw, and combination of CM-118 and rapamycin. Statistical analysis: *P < 0.05; **P < 0.01.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.