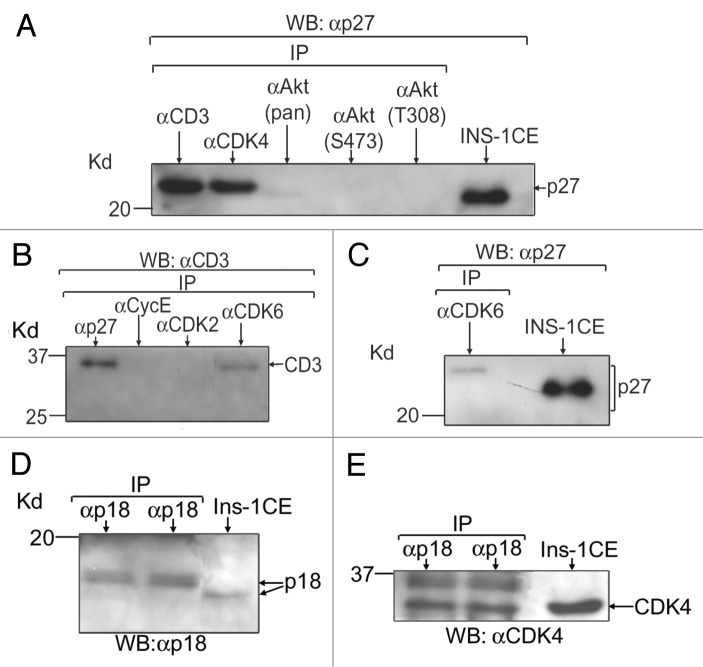
Figure 7. p27(Kip1) and p18(Ink4c) interact with the positive cell cycle regulators in adult human islets. Binding abilities of p27(Kip1) and p18(Ink4c) with the positive cell cycle regulators were assessed by IP and WB assays using lysates from isolated adult human islets (islet purity: 80–90% and age of donors: 40–70 y). For each experiment (n = 3 independent experiments), total protein extracts from isolated human islets were prepared following the procedure as described before9 and equal amounts (40–50 μg) of such lysates for each sample were IP using the antibodies as mentioned in panels (A–E). Proteins bound to agarose beads were boiled in 1X Laemmli sample buffer containing βME and run in SDS-PAGE and then transferred to membranes for WB assays as described before.9 The antibodies used for immunoblotting are mentioned in (A–E). In (A), anti-cyclinD3 and anti-CDK4 antibodies were used to pull down p27(Kip1). The 3 antibodies, anti-Akt (pan), anti-Akt (S473) and anti-Akt (T308), were unable to pull down any detectable levels of p27(Kip1), indicating an inability or low levels of interaction with p27(Kip1). In (B), anti-p27(Kip1) and anti-CDK6 antibodies were used to pull down cyclinD3. The 2 antibodies, anti-cyclinE and anti-CDK2, were used as negative controls. In (C), anti-CDK6 antibody was employed to pull down p27(Kip1). In (D and E), anti-p18(Ink4c) antibodies were used to pull down p18(Ink4c) and CDK4, respectively. Controls without primary antibodies were included to examine any non-specific bindings (data not shown since non-specific bindings were absent). In (E), the band with slower mobility (in duplicate lanes) indicates a phosphorylated from of CDK4 that remains bound with p18(Ink4c). Total cell extracts (CE) from rodent β-cells (INS-1/Ins-1) were applied as positive controls. Here, the abbreviated forms, p27, CD3, CycE and p18, stand for p27(Kip1), cyclinD3, cyclin E, and p18(Ink4c), respectively.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.