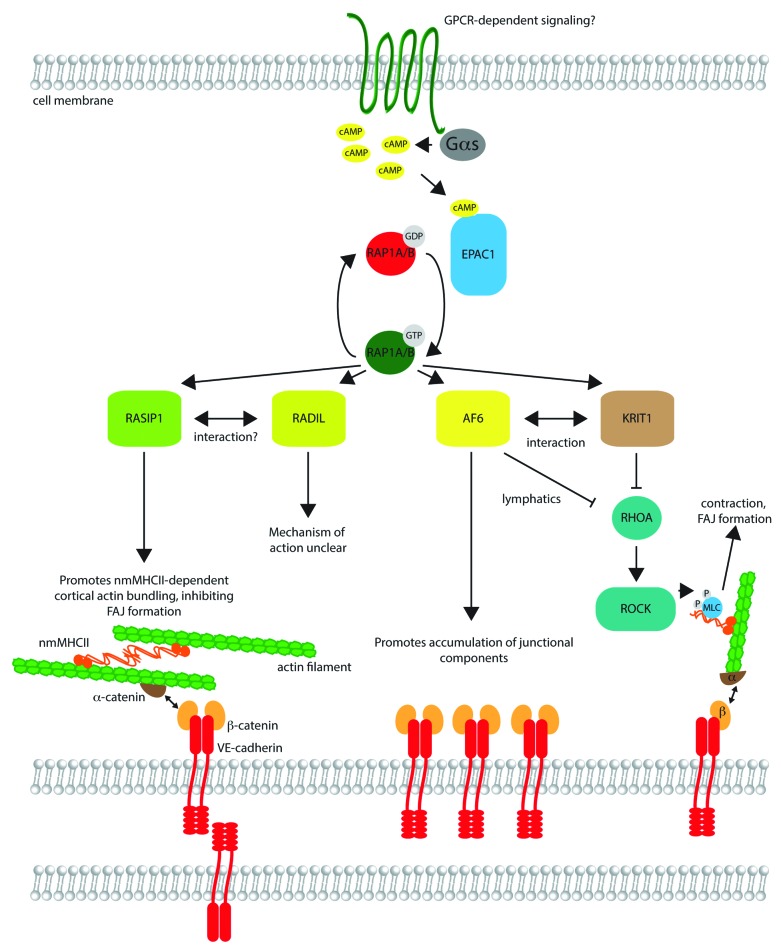
Figure 1. Signaling by RAP1 effectors in endothelial barrier control. Upstream signaling at the cell membrane, potentially mediated by G-protein coupled receptors (GPCRs), triggers activation of adenylyl cyclase via Gαs, resulting in formation of cyclic AMP (cAMP). cAMP binds to EPAC1, a guanine nucleotide exchange factor, which catalyzes exchange of GDP on RAP1A/B for GTP, activating the small G protein. Active RAP1A/B may bind to effectors such as RASIP1, RADIL, AF6, and KRIT1, in many cases triggering their movement to the cell cortex (not shown). RASIP1 promotes bundling of cortical actin, cross-linked by non-muscle myosin heavy chain II (nmMHCII). The cortical actin bundles are linked to transmembrane VE-cadherin molecules through α-catenin and β-catenin, cytosolic adaptor proteins. Assembly of this actin network may inhibit the formation of focal adherens junctions, comprised of cadherin–catenin complexes linked to longitudinal stress fibers. AF6 (also known as afadin) promotes accumulation of junctional components, and binds directly to β-catenin, as well as KRIT1. AF6 and KRIT1 suppress RHOA activation in certain cell types (e.g., AF6 in lymphatic endothelial cells). Inhibition of RHOA reduces phosphorylation of myosin light chain (MLC) by Rho kinase (ROCK), reducing contraction and potentially FAJ formation. The role of RADIL is less clear, but it may partner with RASIP1 and also inhibit RHOA signaling in some contexts.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.