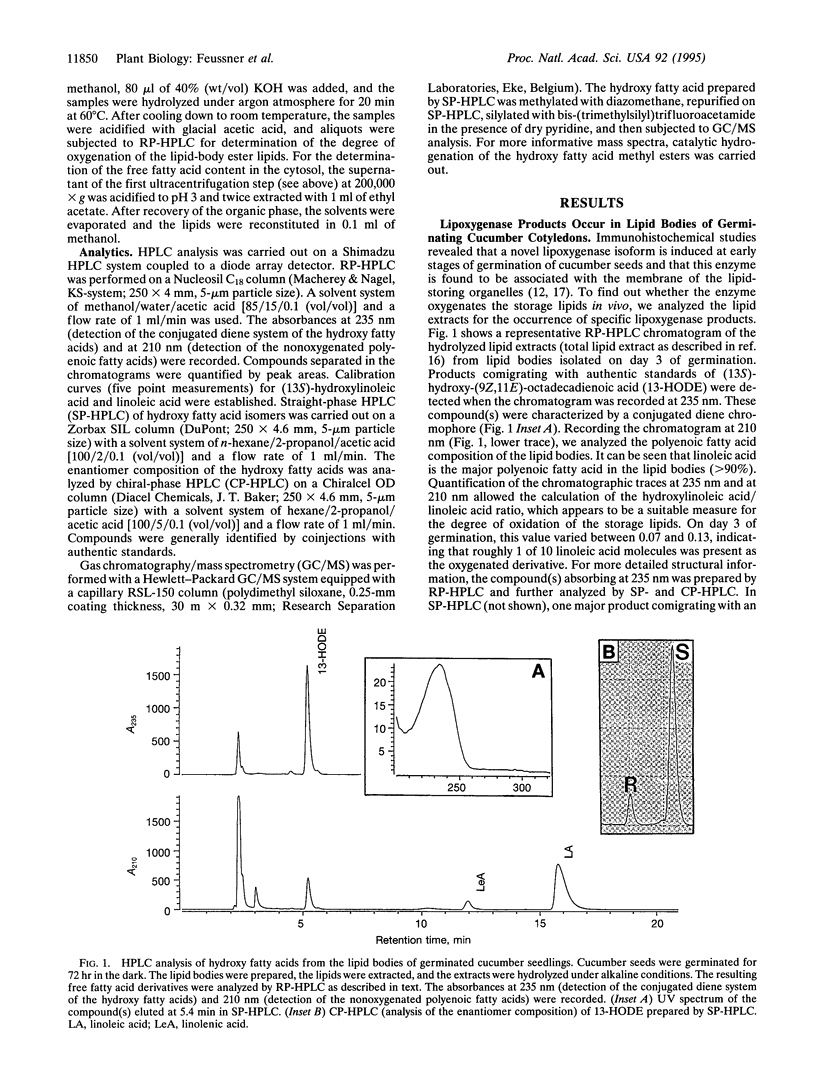
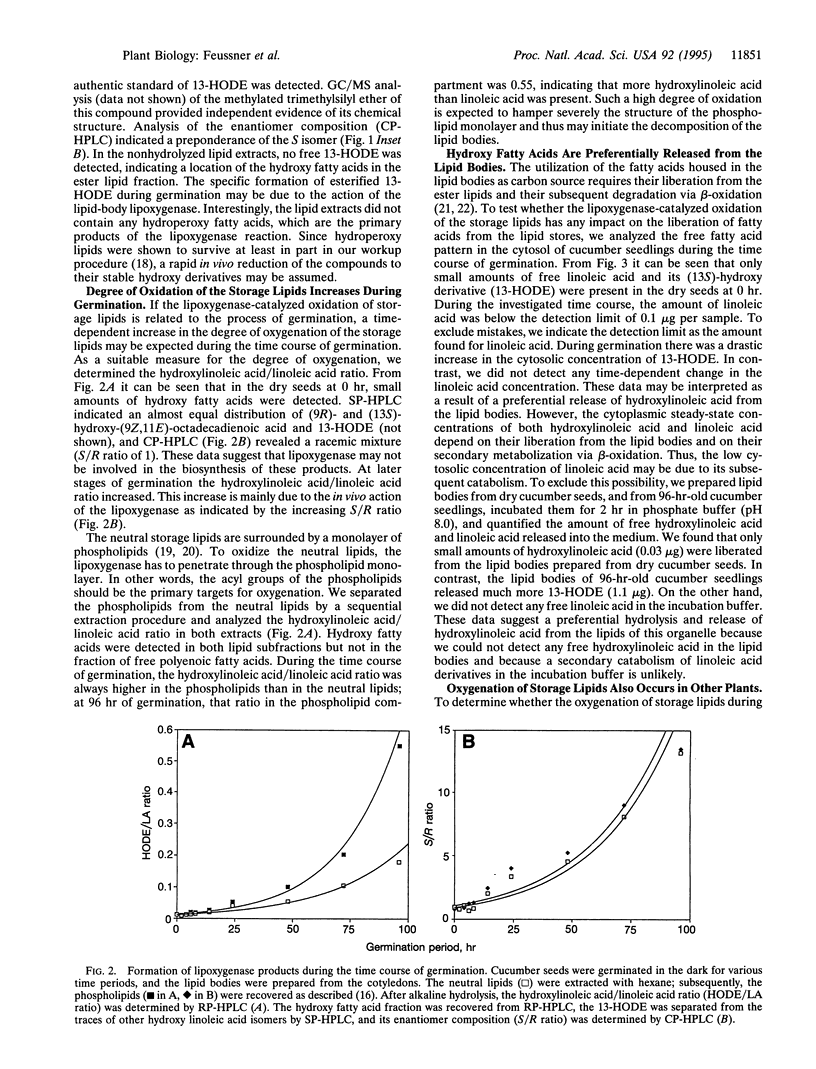
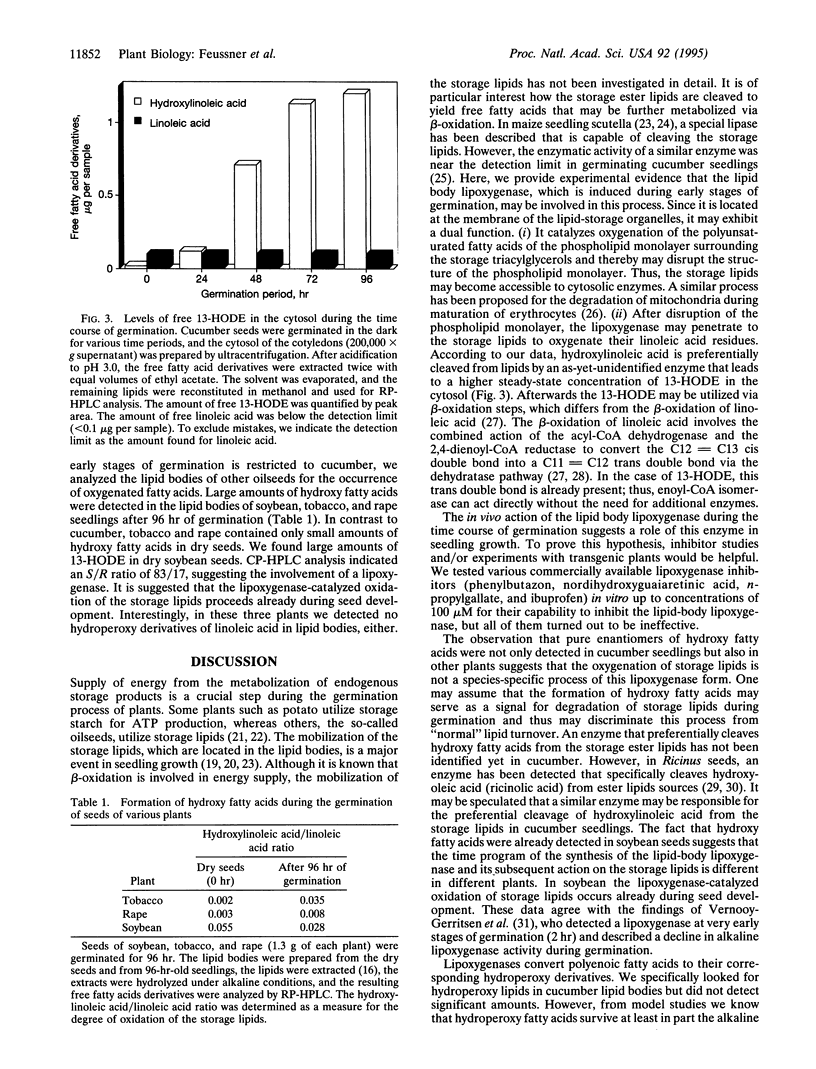
Abstract
The etiolated germination process of oilseed plants is characterized by the mobilization of storage lipids, which serve as a major carbon source for the seedling. We found that during early stages of germination in cucumber, a lipoxygenase (linoleate: oxygen oxidoreductase, EC 1.13.11.12) form is induced that is capable of oxygenating the esterified fatty acids located in the lipid-storage organelles, the so-called lipid bodies. Large amounts of esterified (13S)-hydroxy-(9Z,11E)-octadecadienoic acid were detected in the lipid bodies, whereas only traces of other oxygenated fatty acid isomers were found. This specific product pattern confirms the in vivo action of this lipoxygenase form during germination. Lipid fractionation studies of lipid bodies indicated the presence of lipoxygenase products both in the storage triacylglycerols and, to a higher extent, in the phospholipids surrounding the lipid stores as a monolayer. The degree of oxygenation of the storage lipids increased drastically during the time course of germination. We show that oxygenated fatty acids are preferentially cleaved from the lipid bodies and are subsequently released into the cytoplasm. We suggest that they may serve as substrate for beta-oxidation. These data suggest that during the etiolated germination, a lipoxygenase initiates the mobilization of storage lipids. The possible mechanisms of this implication are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Behrends W., Thieringer R., Engeland K., Kunau W. H., Kindl H. The glyoxysomal beta-oxidation system in cucumber seedlings: identification of enzymes required for the degradation of unsaturated fatty acids. Arch Biochem Biophys. 1988 May 15;263(1):170–177. doi: 10.1016/0003-9861(88)90625-x. [DOI] [PubMed] [Google Scholar]
- Blée E., Wilcox A. L., Marnett L. J., Schuber F. Mechanism of reaction of fatty acid hydroperoxides with soybean peroxygenase. J Biol Chem. 1993 Jan 25;268(3):1708–1715. [PubMed] [Google Scholar]
- Bowman V. B., Huang V., Huang A. H. Expression of lipid body protein gene during maize seed development. Spatial, temporal, and hormonal regulation. J Biol Chem. 1988 Jan 25;263(3):1476–1481. [PubMed] [Google Scholar]
- Feussner I., Kindl H. A lipoxygenase is the main lipid body protein in cucumber and soybean cotyledons during the stage of triglyceride mobilization. FEBS Lett. 1992 Feb 24;298(2-3):223–225. doi: 10.1016/0014-5793(92)80062-l. [DOI] [PubMed] [Google Scholar]
- Feussner I., Kühn H. The lipid body lipoxygenase from cucumber seedlings exhibits unusual reaction specificity. FEBS Lett. 1995 Jun 19;367(1):12–14. doi: 10.1016/0014-5793(95)00531-d. [DOI] [PubMed] [Google Scholar]
- Funk M. O., Carroll R. T., Thompson J. F., Dunham W. R. The lipoxygenases in developing soybean seeds, their characterization and synthesis in vitro. Plant Physiol. 1986 Dec;82(4):1139–1144. doi: 10.1104/pp.82.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner H. W. Recent investigations into the lipoxygenase pathway of plants. Biochim Biophys Acta. 1991 Jul 30;1084(3):221–239. doi: 10.1016/0005-2760(91)90063-n. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Fahlstadius P. On the Specificity of a Fatty Acid Epoxygenase in Broad Bean (Vicia faba L.). Plant Physiol. 1992 Jul;99(3):987–995. doi: 10.1104/pp.99.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Hamberg G. Hydroperoxide-dependent epoxidation of unsaturated fatty acids in the broad bean (Vicia faba L.). Arch Biochem Biophys. 1990 Dec;283(2):409–416. doi: 10.1016/0003-9861(90)90662-i. [DOI] [PubMed] [Google Scholar]
- Kato T., Ohta H., Tanaka K., Shibata D. Appearance of new lipoxygenases in soybean cotyledons after germination and evidence for expression of a major new lipoxygenase gene. Plant Physiol. 1992 Jan;98(1):324–330. doi: 10.1104/pp.98.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Belkner J., Wiesner R. Subcellular distribution of lipoxygenase products in rabbit reticulocyte membranes. Eur J Biochem. 1990 Jul 20;191(1):221–227. doi: 10.1111/j.1432-1033.1990.tb19113.x. [DOI] [PubMed] [Google Scholar]
- Murphy D. J. Storage lipid bodies in plants and other organisms. Prog Lipid Res. 1990;29(4):299–324. [PubMed] [Google Scholar]
- Schewe T., Kühn H. Do 15-lipoxygenases have a common biological role? Trends Biochem Sci. 1991 Oct;16(10):369–373. doi: 10.1016/0968-0004(91)90153-m. [DOI] [PubMed] [Google Scholar]
- Stahl U., Banas A., Stymne S. Plant Microsomal Phospholipid Acyl Hydrolases Have Selectivities for Uncommon Fatty Acids. Plant Physiol. 1995 Mar;107(3):953–962. doi: 10.1104/pp.107.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranbarger T. J., Franceschi V. R., Hildebrand D. F., Grimes H. D. The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell. 1991 Sep;3(9):973–987. doi: 10.1105/tpc.3.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooy-Gerritsen M., Bos A. L., Veldink G. A., Vliegenthart J. F. Localization of lipoxygenases 1 and 2 in germinating soybean seeds by an indirect immunofluorescence technique. Plant Physiol. 1983 Oct;73(2):262–267. doi: 10.1104/pp.73.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim Biophys Acta. 1992 Oct 30;1128(2-3):117–131. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]


