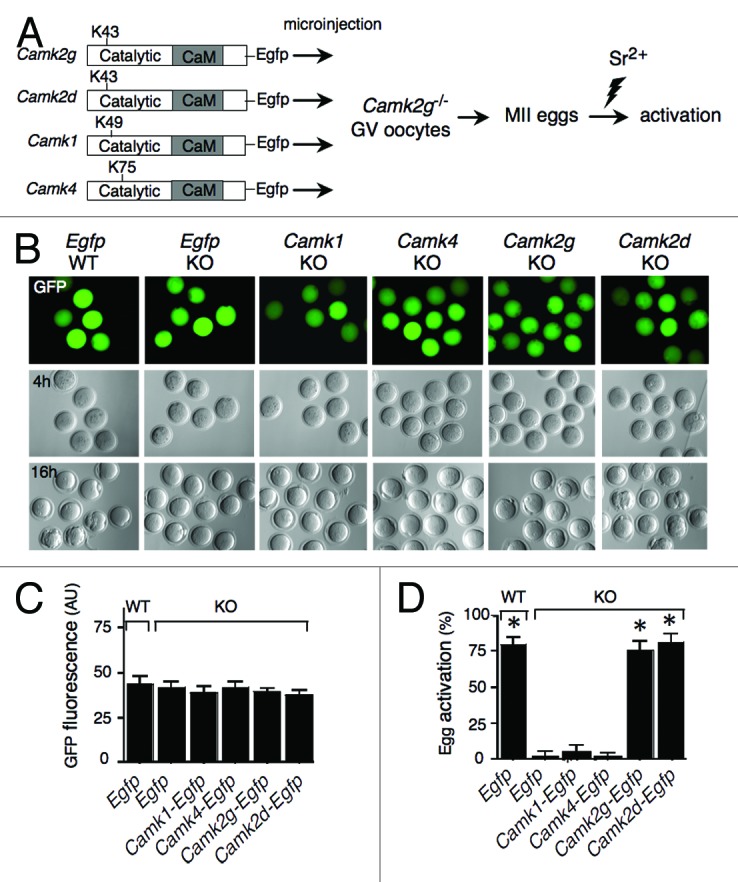
Figure 1. CaMKIIγ and δ, but not CaMKI or CaMKIV, can trigger MII exit in mouse eggs. (A) Schematic representation of the experimental procedure. cRNAs encoding EGFP or EGFP fused to different CaM kinases were microinjected into Camk2g−/− (KO) GV oocytes. As a control, Egfp cRNA was also injected into wild-type (WT) oocytes. Indicated are the catalytic and Ca2+/CaM-binding (CaM) domains, as well as the catalytic site (K43, K49, or K75) of each CaM kinase. Injected oocytes were matured in vitro, parthenogenetically activated with 10 mM SrCl2 (Sr2+) for 3 h, and scored for activation by pronucleus formation. (B) Paired EGFP fluorescence (top) and DIC images following injection of the cRNAs depicted in (A) into Camk2g−/− (KO) or Camk2g+/+ (WT) GV oocytes. The concentration of the different CaMK constructs was adjusted to yield similar levels of GFP fluorescence 4 h after injection (upper panel). The lower panel shows the presence of pronuclei in the control and Camk2g- and Camk2d-injected eggs 16 h after activation. A representative image of 4 experiments is shown. Magnification: 200×. (C) Comparable expression levels of the different constructs. The concentration of the different CaMK constructs was adjusted to yield similar levels of GFP fluorescence upon injection. The fluorescence was quantified using ImageJ software. The experiment was conducted 4 times, and the data are expressed as the mean ± SEM; at least 6 oocytes were analyzed in each group. (D) Egg activation, as measured by pronuclear formation, in the different groups 16 h after exposure to 10 mM SrCl2. The data are expressed as the mean ± SEM. Statistical analysis was performed using ANOVA. *P < 0.0001 vs. KO eggs injected with Egfp cRNA.
