Highlights
-
•
CD9+ germinal center B cells are in close proximity to follicular dendritic cells (FDC) in vivo.
-
•
CD9 improves the survival of GC-B cells in the presence of FDC in vitro.
-
•
CD9 enhances the binding of GC-B cells to soluble VCAM-1.
-
•
CD9 on GC-B cells may have a role in enhancing the affinity of VLA4 to VCAM-1 on FDC.
Abbreviations: Ag, antigen; BCR, B cell receptor; GC, germinal center; FDC, follicular dendritic cells; PC, plasma cells
Keywords: CD9, Germinal center B cells, Follicular dendritic cells, VLA4, VCAM-1, Apoptosis
Abstract
The germinal center (GC) is a dynamic microenvironment where antigen (Ag)-activated B cells rapidly expand and differentiate, generating plasma cells (PC) that produce high-affinity antibodies. Precise regulation of survival and proliferation of Ag-activated B cells within the GC is crucial for humoral immune responses. The follicular dendritic cells (FDC) are the specialized stromal cells in the GC that prevent apoptosis of GC-B cells. Recently, we reported that human GC-B cells consist of CD9+ and CD9− populations and that it is the CD9+ cells that are committed to the PC lineage. In this study, we investigated the functional role of CD9 on GC-B cells. Tonsillar tissue section staining revealed that in vivo CD9+ GC-B cells localized in the light zone FDC area. Consistent this, in vitro CD9+ GC-B cells survived better than CD9− GC-B cells in the presence of HK cells, an FDC line, in a cell–cell contact-dependent manner. The frozen tonsillar tissue section binding assay showed that CD9+ GC-B cells bound to the GC area of tonsillar tissues significantly more than the CD9− GC-B cells did and that the binding was significantly inhibited by neutralizing anti-integrin β1 antibody. Furthermore, CD9+ cells bound to soluble VCAM-1 more than CD9− cells did, resulting in activation and stabilization of the active epitope of integrin β1. All together, our data suggest that CD9 on GC-B cells contributes to survival by strengthening their binding to FDC through the VLA4/VCAM-1 axis.
1. Introduction
Tetraspanins are a large superfamily of proteins with four highly conserved transmembrane domains [1]. Tetraspanins interact with one another and many other proteins, organize a network of molecular interactions into functional microdomains, and regulate cellular process in which the associated proteins are involved [2,3]. The list of tetraspanin-associated proteins is growing and includes integrins and other adhesion molecules, proteins with Immunoglobulin (Ig) domains, enzymes (e.g., ectopeptidases and metalloproteases), and intracellular signaling molecules (e.g., heterotrimeric G proteins, PI4K, activated PKC) [4]. In immune cells, tetraspanins interact with key leukocyte receptors such as the B cell receptor (BCR) complex, CD4/CD8, MHC molecules, and integrins, and thus modulate leukocyte receptor activation and downstream signaling pathways [5]. Recent studies with mice lacking CD37 and CD81 showed that these tetraspanins on B cells are important in the humoral immune response [5]. CD37 on B cell supports long-lived plasma cell survival by orchestrating the VLA4-AKT signaling axis [6], while CD81 on B cells organizes CD19, which is required for the effective BCR signaling [7]. In contrast, the tetraspanins CD9, CD151, and Tssc6 appeared to be dispensable in the B cell immune response [5]. The functions of tetraspanins in the human B cell-mediated immune response, however, have not been largely explored yet. Recently, we reported that CD9 is expressed in a subset of the human tonsillar germinal center (GC) B cells but not in naïve and memory B cells [8]. The specific expression of CD9 on human GC-B cells in contrast to mouse GC-B cells that do not express CD9 [9] suggests that CD9 on human GC-B cells may have a functional role in the GC microenvironment.
The GC is a specialized microenvironment where high-affinity antibody-secreting plasma cells (PC) and memory B cells are generated. Recently introduced imaging approaches advanced our understanding of the germinal center as a highly dynamic structure in which B cells move around and interact with other neighboring cells to produce B cells with high affinity [10,11]. Following the BCR stimulation by antigens, B cells migrate into the GC and rapidly proliferate within the GC microenvironment created by follicular dendritic cells (FDC) and T cells. During this phase of rapid proliferation, antibody diversification processes such as somatic hypermutation and class-switch recombination take place. Somatic hypermutation introduces point mutations into the IgV gene and class-switch recombination alters the effector function of the antibody by switching the constant region of IgM to the IgG, IgE, or IgA isotypes. Subsequently, newly mutated cells expressing high-affinity BCR against antigen on FDC are selected and subsequently present this antigen to T cells for further selection. During the selection process by BCR, the B cells are programmed to die unless survival signals are provided from FDC [12]. While several adhesion molecules and soluble factors from FDC have been reported as promoting GC-B cell survival [13], physical interactions appeared to be necessary for FDC-mediated GC-B cell survival [14]. Adhesion molecules such as ICAM-1 and VCAM-1 on FDC mediate the anti-apoptotic FDC effect on GC-B cells [15].
Here, we investigated the functional role of CD9 on B cells within the GC microenvironment using human tonsillar GC-B cells, which consists of CD9− and CD9+ populations [8]. CD9 expression on human GC-B cells enhances the affinity of VLA4 to VCAM-1 on FDC. The strong adhesion of GC-B cells to FDC is promoted by CD9, which in turn contributes to better survival of GC-B cells.
2. Results
2.1. CD9+ GC-B cells reside in the FDC area of the light zone of the GC
The interactions between GC-B cells and FDC are essential for GC-B cell survival and differentiation [13]. Since CD9 is implicated in the cell–cell interaction [16–18], we hypothesized that CD9 on GC-B cells may play a role in the interaction between GC-B cells and FDC. To address this question, we first determined whether CD9+ GC-B cells were located in the FDC area in vivo. Previously, we demonstrated that tonsillar GC-B cells were composed of CD9+ and CD9− populations and that this ratio was various from donor to donor [8]. Consistent with our previous observation, a portion of CD10+ GC-B cells expressed CD9 when tonsillar tissue sections were stained with anti-CD10 and anti-CD9 antibodies (Supplemental Fig. 1). The tonsillar tissue sections were further stained with antibodies against FDC markers in combination with anti-CD9 to determine whether CD9+ GC-B cells were situated in the FDC area (Fig. 1A and B). Among FDC markers, CD23 and CD54 were selected because CD23+ FDC and CD54+ FDC reside in the light zone of the GC, where FDC are abundant [19]. CD9+ cells were localized both in the apical and basal light zones of the GC where CD23+ and CD54+ FDC, respectively, reside [20]. Furthermore, the majority of CD9+ cells did not express Ki-67, a marker for centroblasts of the dark zone area (Fig. 1C). Although CD9+ and Ki-67+ cells were not mutually exclusive, the staining pattern showed that CD9+ cells and Ki-67+ cells occupied two distinctive areas, confirming that CD9+ cells were located in the light zone of the GC. The stainings were specific because no expression was observed in tonsillar tissue sections stained with corresponding control antibodies (data not shown). These data suggest that CD9+ GC-B cells are in close contact with FDC in vivo.
Fig. 1.
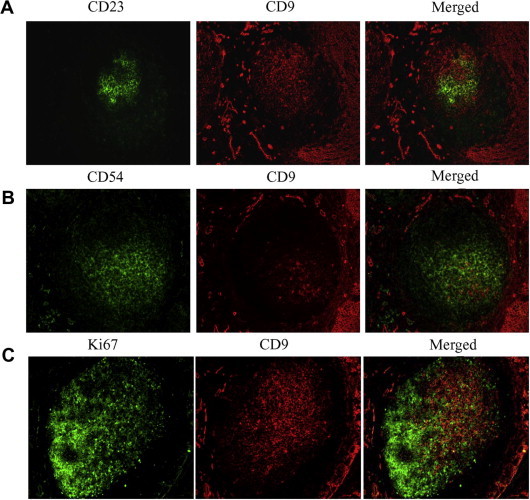
Immunofluorescent staining for CD9 in the germinal centers of human tonsils. The frozen tonsillar tissue sections were stained with anti-CD9 (A–C, red) in combination with anti-CD23 (A, green), anti-CD54 (B, green), and anti-Ki67 (C, green). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.2. CD9+ GC-B cells survive better than CD9− GC-B cells in the presence of FDC/HK cells in a cell–cell contact-dependent manner
The in vivo location of CD9+ GC-B cells in the FDC area prompted us to determine whether CD9 has a functional role in the interaction between GC-B cells and FDC. To address this question in vitro, we utilized an FDC line, HK cells [21]. Like FDC, HK cells prevented GC-B cells from spontaneous apoptosis [22,23]. Therefore, we hypothesized that CD9 expression on GC-B cells may provide a survival advantage by interacting with FDC/HK cells. GC-B cells were first isolated and CD9+ and CD9− GC-B cell populations were further separated using a MACS column. Each population was cultured with CD40L in the presence or absence of HK cells for 24 h, and apoptosis was measured. As shown in Fig. 2A and B, CD9+ GC-B cells consistently survive better than CD9− cells in the presence of HK cells. However, the percentage of Annexin V+ cells (early and late apoptotic cells) in CD9+ and CD9− GC-B cells was comparable in the absence of HK cells. These data suggested that CD9− and CD9+ cells were not intrinsically different in propensity to apoptosis but rather CD9+ cells survive better while in interaction with FDC/HK cells. Greater survival of the CD9+ population compared to the CD9− population in the presence of FDC/HK cells was not attributed to the anti-CD9 antibody bound to CD9+ GC-B cells during the separation procedure because the anti-CD9 antibody (clone 10E5) did not affect cell survival when added in the culture of GC-B cells (Supplemental Fig. 2).
Fig. 2.
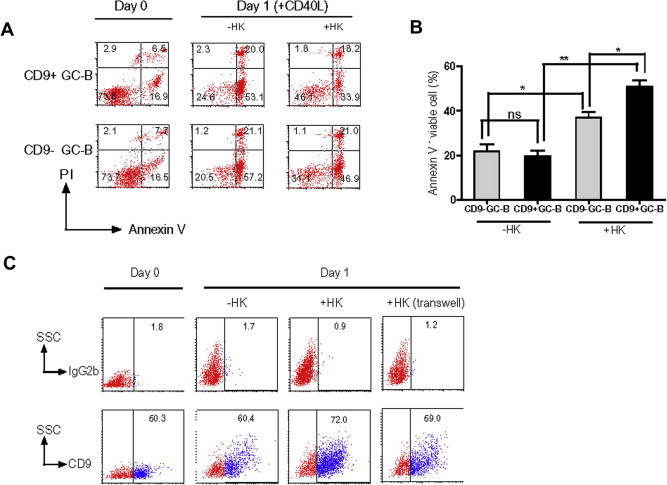
CD9+ GC-B cells survive better than CD9− GC-B cells in the presence of FDC/HK cells. (A and B) CD9+ and CD9− GC-B cells were cultured with CD40L in the presence or absence of HK cells for 24 h, and Annexin V-FITC and PI binding were measured by flow cytometry to assess apoptosis. (C) GC-B cells were cultured with CD40L in the presence or absence of HK cells for 24 h. In some experiments, GC-B cells were separated from HK cells by transwell inserts. At the end of the culture, cells were stained with either anti-CD9 or isotype control antibodies and analyzed by flow cytometry. The percentage of the surviving CD9+ GC-B cells was determined.
To be more secure in our conclusion that the anti-CD9 antibody bound to CD9+ GC-B cells during separation procedure did not enhance CD9+ GC-B cell survival in the presence of HK cells, we assessed HK dependency of CD9+ GC-B cells with another method. GC-B cells without separation by CD9 were cultured with CD40L in the presence or absence of HK cells and 24 h later, the surviving GC-B cells were examined for CD9 expression. Regardless of donors, the percentage of the surviving CD9+ GC-B cells was consistently higher in the presence of HK cells than in the absence of HK cells (Fig. 2C and Table 1), confirming that CD9+ GC-B cells survived better than CD9− GC-B cells with FDC/HK cells. To further determine whether CD9+ GC-B cells survive better than CD9− GC-B cells in the presence of HK cells through cell–cell contacts, we performed transwell experiments (Fig. 2C and Table 1). When GC-B cells were separated from HK cells by transwell inserts, the percentage of surviving CD9+ GC-B cells decreased to a level similar to that of CD9+ GC-B cells in the absence of HK cells, suggesting that direct contact with FDC/HK cells is essential for the CD9-mediated survival advantage in CD9+ GC-B cells. The physical contact of CD9+ GC-B cells and FDC/HK cells appeared not to be mediated via ECM because fibronectin and laminin-produced by FDC/HK cells could not rescue the CD9+ GC-B cell population (Supplemental Fig. 3).
Table 1.
Percentage of surviving CD9+ GC-B cells in the presence or absence of HK cells.
| Day of culture | CD9+ GC-B cells (%) |
|||||
|---|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 5 | ||
| Day 0 | 58.5 | 26.2 | 48.5 | |||
| Day 1 | −HK | 30.5 | 39.9 | 21.4 | 26.1 | 58.7 |
| +HK | 59.0 | 54.2 | 41.0 | 33.5 | 71.1 | |
| +HK (transwell) | 27.3 | 22.8 | 57.8 | |||
GC-B cells were cultured with CD40L in the presence or absence of HK cells for 24 h. In some experiments, GC-B cells are separated from HK cells by transwell inserts. At the end of the culture, cells were stained with anti-CD9 or isotype control antibody and analyzed by flow cytometry. Percentage of the surviving CD9+ GC-B cells was determined.
2.3. Binding of CD9+ GC-B cells to the tonsillar tissue sections is inhibited by anti-integrin β1 antibody
Numerous studies have shown that CD9 forms a complex with integrin β1 and promotes integrin-based functions [24–28]. In addition, the interaction between GC-B cells and FDC through VLA4/VCAM-1 is essential for GC-B cell survival [15]. Hence, we hypothesized that the CD9 on GC-B cells contributes to FDC-mediated GC-B cell survival through VLA4/VCAM-1 interaction. To determine whether the CD9 on GC-B cells is involved in the interaction with FDC, we performed a frozen section binding assay, which was reportedly useful in showing that human B cells adhere to FDC in the GC through VLA4–VCAM1 interaction [29,30]. First, tonsillar tissue sections were incubated with Calcein-AM-labeled CD9+ or CD9− GC-B cells for 30 min. Then, the bound cells were observed under a fluorescent microscope after washing. As shown in Fig. 3Ai and Aii, significantly more CD9+ GC-B cells bound to the GC area of tonsillar tissues than did CD9− GC-B cells. The greater binding of CD9+ GC-B cells to the tonsillar tissue sections was not attributable to the anti-CD9 antibody bound to CD9+ GC-B cells during separation procedure because the anti-CD9 antibody did not enhance unseparated GC-B cell binding when incubated before applied to the tonsillar tissue (Supplemental Fig. 4). Next, we determined whether the binding of CD9+ GC-B cells to the GC area of tonsillar tissues was mediated by integrin. When CD9+ GC-B cells were preincubated with a neutralizing anti-integrin β1 antibody, their binding to tonsillar tissues was dramatically inhibited compared to preincubation with an isotype control antibody (Fig. 3Aiii and Aiv), suggesting that integrin β1 is involved in CD9+ GC-B cell binding to FDC.
Fig. 3.
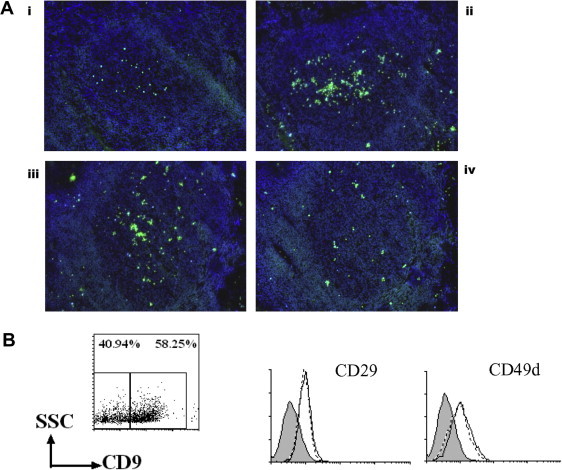
The binding of CD9+ GC-B cells to the tonsillar tissue section is inhibited by the anti-integrin β1 antibody. (A) CD9+ and CD9− GC-B cells were internally labeled with Calcein-AM and then applied to the tonsillar tissue sections. Slides were then examined under a fluorescent microscope: (i) CD9− GC-B cells, (ii) CD9+ GC-B cells, (iii) CD9+ GC-B cells preincubated with an isotype control antibody, and (iv) CD9+ GC-B cells preincubated with an anti-integrin β1 antibody. Representative data from two independent experiments are shown. (B) GC-B cells were stained with anti-CD9, anti-CD29, and CD49d. Histograms with broken and solid lines indicate the expression levels of CD29 and CD49d in CD9− GC-B cells and CD9+ GC-B cells, respectively. Filled histograms indicate isotype control.
To determine whether the quantitative difference in VLA4 expression of CD9− and CD9+ GC-B cells accounts for their difference in GC binding, we examined VLA4 expression in the two populations (Fig. 3B). FACS staining revealed no quantitative difference in either integrin α4 (CD49d, clone 9F10) or integrin β1 (CD29, clone Mar4) expression between the two populations. This finding was confirmed using additional anti-CD49d (clones 2B4) and anti-CD29 (clones P5D2 and TDM29) antibodies (data not shown). These data suggest that the two populations may be different in affinity or avidity of VLA4 for its ligand, VCAM-1.
2.4. CD9 may facilitate VCAM-1 binding by enhancing the exposure of high-affinity conformational epitope of VLA4
To determine whether CD9 is involved in the integrin-mediated binding of CD9+ GC-B cells, we attempted to knock down CD9 in CD9+ GC-B cells. However, we were unsuccessful because of the fragile nature of GC-B cells. Hence, we took advantage of a human B lymphoma cell line of GC origin, L3055 cells [31]. We previously reported the differential CD9 expression in two clones from the parent L3055 cells [32]. Analogous to GC-B cells, the CD9+ L3055-12 clone is more FDC/HK cell-dependent for its survival than CD9− L3055-33 [32]. FACS staining revealed that both CD9+ L3055-12 and CD9− L3055-33 expressed CD29 and CD49d with no quantitative difference between them, just as GC-B cells did (Fig. 4A).
Fig. 4.
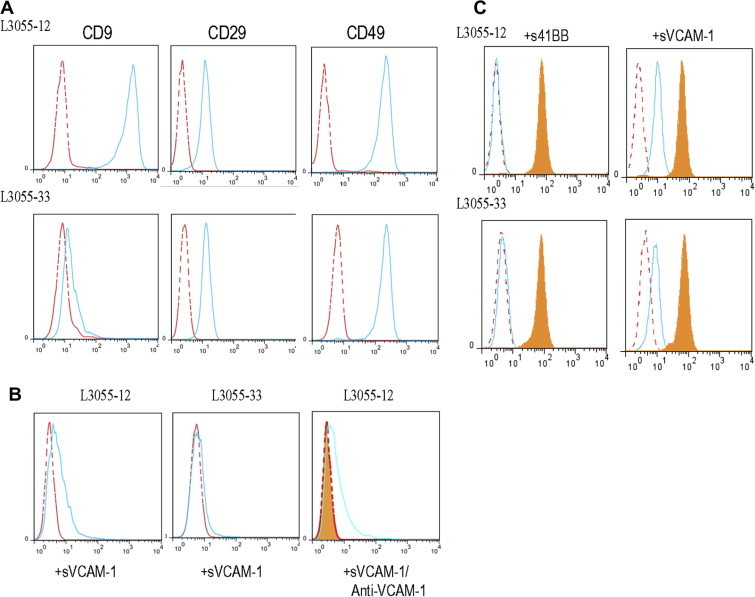
CD9 is involved in optimal VCAM-1 binding and modulation of the integrin activation state. (A) L3055-12 and L3055-33 were stained with anti-CD9, anti-CD29, and CD49d. Histograms with broken and solid lines indicate isotype control and specific antibody, respectively. (B) The binding of soluble VCAM-1-Fc (10 μg/mL) by L3055-12 and L3055-33 was measured by flow cytometry. Competition experiments were performed to confirm the binding specificity by incubating neutralizing anti-VCAM-1 with VCAM-1-Fc for 30 min before being added to L3055-12 cells. Histograms with broken and solid lines indicate the binding to 41BB-Fc (negative control) and VCAM-1-Fc, respectively. The filled histogram indicates the binding to VCAM-1-Fc after the preincubation with anti-VCAM-1. (C) L3055-12 and L3055-33 were stained with anti-CD29 (clone MAR4, total CD29 expression, filled histograms) and anti-CD29 (clone HUTS21, activated form of CD29 expression, empty histograms) following incubation with 41BB-Fc (negative control) and VCAM-1-Fc. Histograms with a broken line indicate the isotype control.
First, we compared CD9+ L3055-12 to CD9− L3055-33 for the binding capacity to soluble VCAM-1, which is known to bind to only high-affinity VLA4 integrin [33]. As shown in Fig. 4B, the CD9+ L3055-12 clone bound to soluble VCAM-1 greater than did the CD9− L3055-33 clone. This binding was specific because the binding of L3055-12 clone to soluble VCAM-1 was abrogated when soluble VCAM-1 was pre-incubated with anti-VCAM-1 antibody. Next, we attempted to determine whether the greater binding of CD9+ L3055-12 to VCAM-1 is attributed to the high-affinity conformational state of VLA4. It has been reported that VLA4 exposes a certain conformational epitope called hybrid domain epitope, upon ligand occupation [34]. The exposure of this epitope, recognized by mAb HUTS21 [35], can be used to determine the VLA4 ligand binding affinity [36]. As expected, mAb HUT21 binding was not detected either in the CD9+ L3055-12 clone or the CD9− L3055-33 clone when the cells were incubated with a negative control ligand, 41BB. However, the epitope was readily detectable after incubation with soluble VCAM-1 (Fig. 4C). The HUT21 mAb binding was quantitatively different between the two clones. CD9+ L3055-12 showed significantly higher binding than CD9− L3055-33 did (Fig. 4C). Total CD29 expression did not change after binding of soluble VCAM-1 (Fig. 4C). All together, these data suggest that CD9 on B cells facilitates VCAM-1 binding by enhancing exposure of high-affinity conformational epitope of VLA4.
3. Discussion
Tetraspanins play a role as molecular facilitators, enhancing the function of the associated molecules [1]. Although it has been reported that tetraspanin CD9 is associated with integrins and facilitates the integrin-mediated functions [24–28], this is the first report suggesting that CD9 may contribute to VLA4-mediated VCAM-1 binding in human mature B cells. Previously, the functional role of two other tetraspanins, CD37 and CD81 in VLA4-mediated VCAM-1 binding of murine B cell has been reported [6,37]. Although both molecules increased VLA4 avidity to its ligands, their underlying mechanisms appeared to be slightly different. While CD37 increased VLA4 affinity to its ligand as well as increased the avidity by inducing VLA4 clusters at the ligand contact site [6], CD81 derived high VLA4 avidity to ligand upon ligand binding in an affinity independent manner [37]. Here, we present evidence that CD9 may enhance VLA4-mediated VCAM-1 binding by increasing VLA4 affinity. It seems that CD9 expression facilitates the exposure of high-affinity conformational epitope of VLA4 upon ligand binding. However, we cannot exclude the possibility that CD9 induces VLA4 clusters at the ligand contact site to strengthen the interaction as well.
Overall, it seems that CD9, CD37, and CD81 have redundant functions in enhancing VLA4-mediated adhesion to VCAM-1. Then, how do cells properly utilize different tetraspanins to do the same process? A simple answer would be the differential expression of tetraspanins in cells. Indeed, Barrena et al. reported that human B cells from different developmental stages have unique expression patterns of tetraspanin proteins [38]. Worth mentioning is that human GC-B cells are reported to express CD81 and CD37 homogeneously, different from CD9 [39,40]. Taken together, it is tempting to speculate that CD9 further optimizes VCAM-1 binding by VLA4 that was modulated by CD81 and CD37. The human GC-B cell adhesion to FDCs without CD9 may be transient and weak while CD9 enhances the adhesion of GC-B cells to FDCs in a strong and sustained manner by stabilizing the VLA4–VCAM-1 interaction through conformational changes of VLA4. The strong adhesion mediated by CD9 in turn may contribute to GC-B cell survival. Indeed, we found that CD9+ GC-B cells survived better than CD9− GC-B cells only when FDCs/HK cells are present in the culture. These data strongly suggest that CD9 is involved in the interaction with FDC. Although we presented evidence supporting the involvement of CD9 in VLA4-mediated firm adhesion to VCAM-1 and subsequent survival, our current study has a limitation. It is possible that CD9+ GC-B cells and CD9− GC-B cells have more differences than CD9 expression, which may contribute to different capacities of these two populations in their adhesion and survival. To clarify the direct role of CD9 in the process, searching for neutralizing anti-CD9 antibodies as well as knocking down the CD9 gene in B cells of GC-origin are underway.
In summary, CD9 expression in human GC-B cells may have a functional role in enhancing the affinity of VLA4 to VCAM-1 on FDC in the light zone of the GC. The strong adhesion mediated by CD9 contributes to better survival of GC-B cells and may give a further advantage over the ongoing selection process in the light zone of the GC.
4. Materials and methods
4.1. Antibodies and reagents
FITC- or PE-conjugated anti-CD9 (clone MM2/57) and FITC-conjugated anti-CD29 (clone TDM29) were from Southern Biotech (Birmingham, AL); PE-conjugated anti-CD10, anti-CD23, anti-CD54, anti-CD29 (clones MAR4 and HUTS21), and anti-CD49d (clone 9F10) were from BD Biosciences (San Jose, CA); FITC-conjugated anti-His and rat anti-mouse IgG1 microbeads were from Miltenyi Biotec (Sunnyvale, CA); FITC-conjugated anti-CD29 (clone P5D2) were from Santa Cruz Biotech (Santa Cruz, CA); FITC-conjugated anti-Ki-67 was from Zymed (Carlsbad, CA); Anti-CD49d (clone 2B4) was from R & D Systems (Minneapolis, MN).
Anti-CD9 (clone 10E5) was a kind gift from Dr. J.C. Choe (Department of Microbiology and Immunology, Kangwon National University School of Medicine, Chunchon, Korea). Soluble human CD40L was generously provided by Dr. R. Armitage (Amgen Corporation, Seattle, WA).
4.2. Immunofluorescence
Cryosections of human tonsils (5 μm thick) were air-dried overnight at room temperature and fixed in cold acetone for 10 min. Nonspecific binding of the antibody was blocked by incubating the slides with 1% BSA-PBS for 30 min at room temperature in a humidified chamber. Slides were incubated with anti-CD9-FITC in combination with anti-CD10-PE, anti-CD23-PE, or anti-CD54-PE or with anti-CD9-PE in combination with anti-Ki-67-FITC. Isotype control-FITC and isotype control-PE were used side by side to exclude false positives. Slides were washed and mounted with anti-fade mounting medium (Invitrogen, Carlsbad, CA). Slides were then examined and images collected on a deconvolution microscope (Axiovert 200M; Carl Zeiss Microimaging, Jena, Germany). Images were processed using the SlideBook software (Intelligent Imaging Innovations, Denver, CO).
4.3. Preparation of GC-B cells and apoptosis assay
Tonsils were obtained from the Surgery Department within the Ochsner Clinic Foundation. Informed consent was not required because these materials are considered to be waste generated during surgery by Institutional Review Board. GC-B cells were isolated from the tonsils and further separated by CD9 expression as described previously [8].
For apoptosis assay, CD9+ and CD9− GC-B cells (3–5 × 105 cells/well) were cultured in 12-well plates either in the presence of CD40L (100 ng/mL) alone or in the presence of CD40L plus irradiated FDC/HK cells (4 × 104 cells/well; 5000 rad) for 24 h. HK cells were maintained as described previously [21]. The GC-B cells were collected, stained with Annexin V-FITC and propidium iodide (PI) according to the manufacturer’s protocol (BD Biosciences), and subjected to flow cytometric analysis.
4.4. Flow cytometry
Cells were stained with a panel of antibodies directly conjugated with PE or FITC by incubating at 4 °C for 15 min. After washing with PBS containing 0.2% BSA and 0.1% sodium azide, cells were fixed with 1% paraformaldehyde and analyzed by flow cytometry.
4.5. Frozen section binding assay
In vitro frozen section binding assay was performed as described by Freedman et al. [29,30]. In short, GC-B cells were labeled with 1 μM Calcein AM (Invitrogen) for 15 min at room temperature in PBS. Cells were washed twice with serum containing media and resuspended at 3 × 107 cells/mL. One hundred microliters of the cell suspension were placed onto frozen sections of tonsil and incubated at 37 °C for 30 min. After incubation, slides were fixed in 3% glutaraldehyde in PBS overnight at 4 °C. Slides were washed, mounted with anti-fade mounting medium containing DAPI (Invitrogen), and then examined by fluorescence microscopy. For blocking experiments, cells were preincubated with neutralizing anti-integrin β1 (R & D Systems) or isotype control antibodies (10 μg/mL) for 30 min before applying to tissue sections.
4.6. sVCAM-1 binding and detection of an activated form of CD29
L3055 cell subclones L3055-12 and L3055-33 were maintained as described previously [32]. L3055-12 and L3055-33 (5 × 105 cells per 100 μL of the complete media) were incubated with soluble VCAM-1-His Tag (10 μg/mL, R & D systems) at 37 °C for 30 min. Binding of soluble VCAM-1 was detected by incubating the cells with the FITC-conjugated anti-His antibody for 20 min at 4 °C. As a negative control, the same amount of soluble 41BB-His Tag (R & D systems) was added. In some experiments, soluble VCAM-1 was preincubated with neutralizing anti-VCAM-1 antibody (10 μg/mL, Beckman Coulter, Indianapolis, IN) before incubation with the cells. To detect an activated form of CD29, the cells were incubated with soluble VCAM-1, followed by staining with PE-conjugated anti-CD29 (clone, HUTS21).
4.7. Statistical analysis
Statistical analysis and graphic presentation were carried out with GraphPad Prism 4.0 (GraphPad, La Jolla, CA). Results are presented as means of triplicate assays plus SEM. The statistical significance of differences was determined by Student’s t-test; P < 0.05 was considered significant.
Author contributions
S.O.Y. planned experiments, performed experiments, analyzed data, and wrote the paper; I.Y.L. performed experiments and analyzed data; X.Z. performed experiments and analyzed data; M.C.Z. performed experiments; Y.S.C. planned experiments and contributed reagents.
Conflict of interest
No potential conflicts of interest were disclosed.
Acknowledgements
This work was supported in part by NIH grants R01CA121039 to Y.S.C. and P20GM103501 to S.O.Y.
Appendix A. Supplementary data
This document file contains Supplementary Figs. 1–4.
References
- 1.Maecker H.T., Todd S.C., Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- 2.Boucheix C., Rubinstein E. Tetraspanins. Cell. Mol. Life Sci. 2001;58:1189–1205. doi: 10.1007/PL00000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemler M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 4.Rubinstein E. The complexity of tetraspanins. Biochem. Soc. Trans. 2011;39:501–505. doi: 10.1042/BST0390501. [DOI] [PubMed] [Google Scholar]
- 5.van Spriel A.B. Tetraspanins in the humoral immune response. Biochem. Soc. Trans. 2011;39:512–517. doi: 10.1042/BST0390512. [DOI] [PubMed] [Google Scholar]
- 6.van Spriel A.B. The tetraspanin CD37 orchestrates the alpha4beta1 integrin-Akt signaling axis and supports long-lived plasma cell survival. Sci. Signal. 2012;5:ra82. doi: 10.1126/scisignal.2003113. [DOI] [PubMed] [Google Scholar]
- 7.Mattila P.K. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity. 2013;38:461–474. doi: 10.1016/j.immuni.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Yoon S.O., Zhang X., Lee I.Y., Spencer N., Vo P., Choi Y.S. CD9 is a novel marker for plasma cell precursors in human germinal centers. Biochem. Biophys. Res. Commun. 2013;431:41–46. doi: 10.1016/j.bbrc.2012.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Won W.J., Kearney J.F. CD9 is a unique marker for marginal zone B cells, B1 cells, and plasma cells in mice. J. Immunol. 2002;168:5605–5611. doi: 10.4049/jimmunol.168.11.5605. [DOI] [PubMed] [Google Scholar]
- 10.Allen C.D., Okada T., Tang H.L., Cyster J.G. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 11.Victora G.D., Schwickert T.A., Fooksman D.R., Kamphorst A.O., Meyer-Hermann M., Dustin M.L., Nussenzweig M.C. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manser T. Textbook germinal centers? J. Immunol. 2004;172:3369–3375. doi: 10.4049/jimmunol.172.6.3369. [DOI] [PubMed] [Google Scholar]
- 13.Park C.S., Choi Y.S. How do follicular dendritic cells interact intimately with B cells in the germinal centre? Immunology. 2005;114:2–10. doi: 10.1111/j.1365-2567.2004.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindhout E., Mevissen M.L.C.M., Kwekkeboom J., Tager J.M., deGroot C. Direct evidence that human follicular dendritic cells (FDC) rescue germinal centre B cells from death by apoptosis. Clin. Exp. Immunol. 1993;91:330–336. doi: 10.1111/j.1365-2249.1993.tb05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koopman G., Keehnen R.M.J., Lindhout E., Newman W., Shimizu Y., van Seventer G.A., de Groot C., Pals S.T. Adhesion through the LFA-1 (CD11a/CD18)-ICAM-1 (CD54) and the VLA-4 (CD49d)-VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. J. Immunol. 1994;152:3760–3767. [PubMed] [Google Scholar]
- 16.Longo N., Yanez-Mo M., Mittelbrunn M., de la Rosa G., Munoz M.L., Sanchez-Madrid F., Sanchez-Mateos P. Regulatory role of tetraspanin CD9 in tumor-endothelial cell interaction during transendothelial invasion of melanoma cells. Blood. 2001;98:3717–3726. doi: 10.1182/blood.v98.13.3717. [DOI] [PubMed] [Google Scholar]
- 17.Barreiro O. Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood. 2005;105:2852–2861. doi: 10.1182/blood-2004-09-3606. [DOI] [PubMed] [Google Scholar]
- 18.Ko E.M. Monoclonal antibody to CD9 inhibits platelet-induced human endothelial cell proliferation. Mol. Cells. 2006;22:70–77. [PubMed] [Google Scholar]
- 19.Stein H., Gerdes H., Mason D. The normal and malignant germinal centre. Clin. Haematol. 1982;11:531–559. [PubMed] [Google Scholar]
- 20.Hardie D.L., Johnson G.D., Khan M., MacLennan I.C.M. Quantitative analysis of molecules which distinguish functional compartments within germinal centers. Eur. J. Immunol. 1993;23:997–1004. doi: 10.1002/eji.1830230502. [DOI] [PubMed] [Google Scholar]
- 21.Kim H.-S., Zhang X., Choi Y.S. Activation and proliferation of follicular dendritic cell-like cells by activated T lymphocytes. J. Immunol. 1994;153:2951–2961. [PubMed] [Google Scholar]
- 22.Kim H.-S., Zhang X., Klyushnenkova E., Choi Y.S. Stimulation of germinal center B lymphocyte proliferation by an FDC-like cell line, HK. J. Immunol. 1995;155:1101–1109. [PubMed] [Google Scholar]
- 23.Choe J., Kim H.S., Zhang X., Armitage R.J., Choi Y.S. Cellular and molecular factors that regulate the differentiation and apoptosis of germinal center B cells. Anti-Ig down-regulates Fas expression of CD40 ligand-stimulated germinal center B cells and inhibits Fas-mediated apoptosis. J. Immunol. 1996;157:1006–1016. [PubMed] [Google Scholar]
- 24.Rubinstein E., Le Naour F., Billard M., Prenant M., Boucheix C. CD9 antigen is an accessory subunit of the VLA integrin complexes. Eur. J. Immunol. 1994;24:3005–3013. doi: 10.1002/eji.1830241213. [DOI] [PubMed] [Google Scholar]
- 25.Ziyyat A. CD9 controls the formation of clusters that contain tetraspanins and the integrin alpha 6 beta 1, which are involved in human and mouse gamete fusion. J. Cell Sci. 2006;119:416–424. doi: 10.1242/jcs.02730. [DOI] [PubMed] [Google Scholar]
- 26.Kotha J., Longhurst C., Appling W., Jennings L.K. Tetraspanin CD9 regulates beta 1 integrin activation and enhances cell motility to fibronectin via a PI-3 kinase-dependent pathway. Exp. Cell Res. 2008;314:1811–1822. doi: 10.1016/j.yexcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Powner D., Kopp P.M., Monkley S.J., Critchley D.R., Berditchevski F. Tetraspanin CD9 in cell migration. Biochem. Soc. Trans. 2011;39:563–567. doi: 10.1042/BST0390563. [DOI] [PubMed] [Google Scholar]
- 28.Pellinen T., Rantala J.K., Arjonen A., Mpindi J.P., Kallioniemi O., Ivaska J. A functional genetic screen reveals new regulators of beta1-integrin activity. J. Cell Sci. 2012;125:649–661. doi: 10.1242/jcs.090704. [DOI] [PubMed] [Google Scholar]
- 29.Freedman A.S. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science. 1990;249:1030–1033. doi: 10.1126/science.1697696. [DOI] [PubMed] [Google Scholar]
- 30.Freedman A.S., Munro J.M., Rhynhart K., Schow P., Daley J., Lee N., Svahn J., Eliseo L., Nadler L.M. Follicular dendritic cells inhibit human B-lymphocyte proliferation. Blood. 1992;80:1284–1288. [PubMed] [Google Scholar]
- 31.Choe J., Li L., Zhang X., Gregory C.D., Choi Y.S. Distinct role of follicular dendritic cells and T cells in the proliferation, differentiation, and apoptosis of a centroblast cell line, L3055. J. Immunol. 2000;164:56–63. doi: 10.4049/jimmunol.164.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Yoon S.O., Zhang X., Freedman A.S., Zahrieh D., Lossos I.S., Li L., Choi Y.S. Down-regulation of CD9 expression and its correlation to tumor progression in B lymphomas. Am. J. Pathol. 2010;177:377–386. doi: 10.2353/ajpath.2010.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose D.M., Cardarelli P.M., Cobb R.R., Ginsberg M.H. Soluble VCAM-1 binding to alpha4 integrins is cell-type specific and activation dependent and is disrupted during apoptosis in T cells. Blood. 2000;95:602–609. [PubMed] [Google Scholar]
- 34.Chigaey A., Sklar L.A. Aspects of VLA-4 and LFA-1 regulation that may contribute to rolling and firm adhesion. Front. Immunol. 2012;3:242. doi: 10.3389/fimmu.2012.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luque A., Gomez M., Puzon W., Takada Y., Sanchez-Madrid F., Cabanas C. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common beta 1 chain. J. Biol. Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 36.Chigaey A., Waller A., Amit O., Halip L., Bologa C.G., Sklar L.A. Real-time analysis of conformation-sensitive antibody binding provides new insights into integrin conformational regulation. J. Biol. Chem. 2009;284:14337–14346. doi: 10.1074/jbc.M901178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feigelson S.W., Grabovsky V., Shamri R., Levy S., Alon R. The CD81 tetraspanin facilitates instantaneous leukocyte VLA-4 adhesion strengthening to vascular cell adhesion molecule 1 (VCAM-1) under shear flow. J. Biol. Chem. 2003;278:51203–51212. doi: 10.1074/jbc.M303601200. [DOI] [PubMed] [Google Scholar]
- 38.Barrena S. Aberrant expression of tetraspanin molecules in B-cell chronic lymphoproliferative disorders and its correlation with normal B-cell maturation. Leukemia. 2005;19:1376–1383. doi: 10.1038/sj.leu.2403822. [DOI] [PubMed] [Google Scholar]
- 39.Luo R.F. CD81 protein is expressed at high levels in normal germinal center B cells and in subtypes of human lymphomas. Hum. Pathol. 2010;41:271–280. doi: 10.1016/j.humpath.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tedoldi S. Selective loss of B-cell phenotype in lymphocyte predominant Hodgkin lymphoma. J. Pathol. 2007;213:429–440. doi: 10.1002/path.2242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document file contains Supplementary Figs. 1–4.


