Abstract
Purpose
Triphala is a combination of three medicinal plants, extensively used in Ayurveda since ancient times. Triphala mouthwash is used in the treatment of periodontal diseases because of its antimicrobial and antioxidant properties. The aim of this study is to compare the efficacy of triphala mouthwash with 0.2% chlorhexidine in hospitalized periodontal disease patients.
Methods
In this double-blind, randomized, multicenter clinical trial, 120 patients were equally divided into three groups. Patients in group A were advised to rinse their mouths with 10 mL of distilled water, group B with 0.2% chlorhexidine, and group C with triphala mouthwash for 1 minute twice daily for two weeks. The plaque index (PI) and the gingival index (GI) were recorded on the first and the fifteenth day.
Results
There was no significant difference when the efficacy of triphala was compared with 0.2% chlorhexidine in hospitalized patients with periodontal disease. However, a statistically significant difference was observed in PI and GI when both group B and group C were compared with group A and also within groups B and C, after 15 days (P<0.05).
Conclusions
The triphala mouthwash (herbal) is an effective antiplaque agent like 0.2% chlorhexidine. It is significantly useful in reducing plaque accumulation and gingival inflammation, thereby controlling periodontal diseases in every patient. It is also cost effective, easily available, and well tolerable with no reported side effects.
Graphical Abstract
Keywords: Chlorhexidine, Gingivitis, Periodontitis, Triphala
INTRODUCTION
Periodontal diseases appear to occur when pathogenic microbial plaque acts on a susceptible host [1]. Supragingival plaque control is fundamental to the prevention and management of periodontal diseases [2], either mechanically or by means of different chemical agents. Mechanical plaque control is a challenge in admitted medically compromised patients because of poor tooth-cleaning habits. Certain teeth surfaces receive minimum attention while tooth brushing. Thus, an adjunctive use of chemical agents has been practiced. Chemical supragingival plaque control has been the subject of extensive research for 3-4 decades now. Various agents that are antimicrobial and prevent the bacterial proliferation phase of plaque development have been introduced to the market [3]. Chlorhexidine (CHX), a cationic bisbiguanide is a gold standard among all mouthwashes [4,5,6,7,8], particularly because of its substantivity and broad-spectrum antibacterial activity [9,10,11]. However, CHX has been reported to have a number of side effects like brown discoloration of teeth, salt taste perturbation, oral mucosal erosions, and enhanced supragingival calculus formation, which limit its long-term use [3,12,13,14].
Triphala is a combination of three medicinal plants, Amalaki Phyllanthus emblica (syn. Emblica officinalis) Phyllanthaceae family, Haritaki (Terminalia chebula) Combretaceae family, and Bahera (Terminalia bellirica) Combretaceae family, and has been extensively used in Ayurveda since ancient times. It is a very useful tool for improving the body's immunity as it readily promotes the body's ability to form antibodies in order to fight any invasion of antigens [15]. Amalaki is an excellent source of vitamin C and also contains carotene, nicotinic acid, D-glucose, D-fructose, riboflavin, empicol, and mucic and phyllemblic acids. Haritaki is used in traditional medicine due to the wide spectrum of pharmacological activities associated with the biologically active chemicals present in this plant. It contains anthraquinone glycoside, chebulinic acid, tannic acid, terchebin, vitamin C, and arachidinic, linoleic, oleic, palmitic, and stearic acids. It inhibits the rate of cell proliferation and cell death in the cancer cell line. Bahera contains chebulagic acid, ellagic acid and its ethyl ester, gallic acid, fructose, galactose, glucose, mannitol, and rhamnose [16]. The antioxidant activity of the extract was indicated by reduced lipid peroxide levels in treated wounds [17]. The dry fruit of the three abovementioned plants and triphala as a whole are easily available in the market and are affordable for all socio-economic strata. Antioxidants present in triphala slow down the process of excess oxidation and protect cells from the damage caused by free radicals [18,19]. This proven activity of these antioxidants is very helpful in modern medicine and the treatment of oxidative stress-related diseases, particularly precancerous/premalignant conditions, and is thus helpful in the prevention of cancers [15,20]. These antioxidants have also been scientifically proven to be a safe and effective medicine against various oral health problems such as bleeding gums, halitosis, and mouth ulcers, and for preventing tooth decay. The major strength of these natural herbs is that thus far, no side effects of their use have been reported [21]. Sushruta Samhita, in its 20th shloka, states that triphala can be used as a gargling agent in dental diseases. Abraham et al. [22] reported the strong inhibitory activity of triphala against the polymorphonuclear leukocytes-type collagenases, particularly matrix metalloproteinase-9, and confirmed the use of triphala in periodontal diseases. Triphala has been reported to have antimicrobial, antiseptic, anti-inflammatory, and antioxidant properties, among others [16,17,23,24,25].
Because of the numerous properties of triphala along with its other advantages like easy availability and cost effectiveness, the present study was undertaken to compare triphala's efficacy with commercially available CHX in hospitalized periodontal disease patients.
MATERIALS AND METHODS
Study design and experimental population
In this double-blind, randomized, multicenter controlled trial, the participants were selected from patients admitted to three different medical hospitals, namely Subdistrict Government Hospital, Kodoli; Mahatma Gandhi Hospital, New Pargaon; and Yeshwant Chavan Hospital, Kodoli, in February 2013. The procedure was conducted according to the 2010 CONSORT (Consolidated Standards of Reporting Trials) guidelines, and an ethical approval was obtained from the Institutional Review Board of Tatyasaheb Kore Dental College. This clinical trial has been registered under ClinicalTrials.gov identifier number: NCT01900535.
The study was powered at 80% to detect a mean difference of 0.5 in the indices after the subjects rinsed their mouths with the assigned mouth rinses and by assuming a 30% within-group change in the primary and secondary outcomes. The minimum required sample size was calculated as 32 patients for each group; to compensate for potential dropouts, it was planned that 40 patients would be recruited to each group.
Hospitalized patients having more than 20 teeth with plaque and calculus and clinical signs of gingival inflammation were screened for the study. Patients seeking any periodontal treatment during the course of study were excluded. Finally, a total of 120 hospitalized patients, 40 from each hospital, in the age group of 20-65 years, were selected in the study. Informed written consent was obtained from the patients after explaining the methodology of the clinical trial. These patients were subsequently divided into three groups, namely, A, B, and C (n=40). Group A was asked to rinse their mouths with distilled water, group B with a 0.2% CHX gluconate mouthwash, and group C with triphala, a herbal mouthwash containing T. chebula, T. bellirica, and E. officinalis. The groups, A, B, and C, were allocated to Subdistrict Government Hospital, Kodoli; Mahatma Gandhi Hospital, New Pargaon; or Yeshwant Chavan Hospital, Kodoli, on the basis of a lottery system. The patients were instructed to rinse their mouths with 10 mL of the assigned mouthwash for 1 minute twice daily for two weeks, without disclosing its nature for blinding the procedure. The patients were instructed to continue with their routine oral hygiene measures and to keep a gap of 30 minutes between tooth brushing and rinsing. The plaque index (PI) and the gingival index (GI) were assessed in each patient on the first and the fifteenth day by two different trained examiners to avoid bias. The two examiners were calibrated prior to the study in order to reduce the inter examiner variability. The primary efficacy outcome was changes in the GI, and the secondary outcome was changes in the PI. Moreover, patients were asked to report any side effect that they experience and were checked for adverse intraoral effects at each appointment.
Preparation of triphala mouthwash
For the preparation of 10 mL of the solution, 10 g of triphala powder containing equal quantities of T. bellirica, T. chebula, and E. officinalis was added to 10 mL of boiling water.
Statistical analysis
All data were entered into a computer, checked for entry errors, and analyzed with a statistical package, IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA). For an intra group comparison of the paired sample, a Student t-test was applied, while for the intergroup comparison, analysis of variance (ANOVA) was applied using the mean difference values of the PI and GI at day 1 and day 15. A P-value of <0.05 was considered statistically significant. Cohen's kappa statistics was applied to standardize the inter- or intra- examiner error variability.
RESULTS
Study population
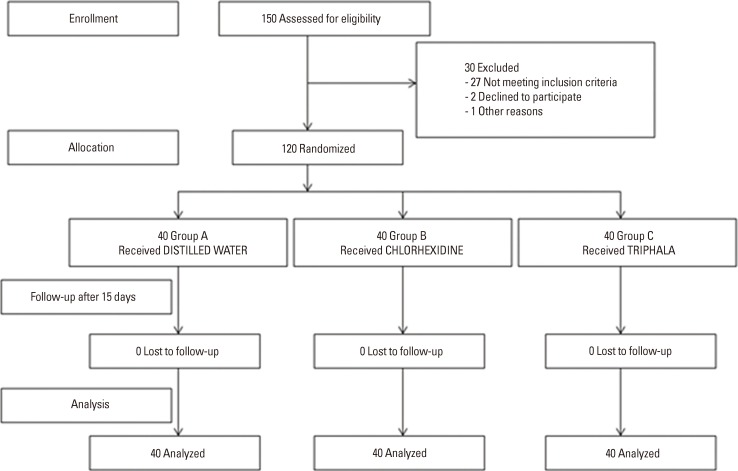
A total of 120 patients (78 males and 42 females) selected from three different hospitals, 40 from each, irrespective of their medical problems, socioeconomic status, and oral habits, completed the study after randomization with no dropouts (Fig. 1). While CHX served as the positive control (group B), placebo-containing distilled water served as the negative control (group A). PI and GI were considered the primary and the secondary end points, respectively. The inter- and intra- examiner variability calculated using Cohen's kappa statistics was 95.3%.
Figure 1.
Study design flow chart. Group A: patients were advised to rinse their mouths with 10 mL of distilled water for 1 minute twice daily for two weeks, group B: patients were advised to rinse their mouths with 0.2% chlorhexidine for 1 minute twice daily for two weeks, group C: patients were advised to rinse their mouths with triphala mouthwash for 1 minute twice daily for two weeks.
Primary and secondary end points: between-group analysis
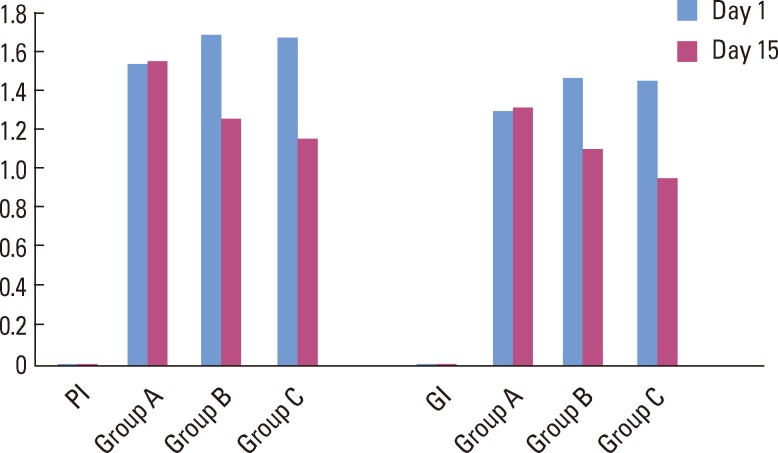
The intergroup comparison of the mean differences in the PI and GI calculated using ANOVA showed no statistically significant difference between group B and group C, but when group B and group C were compared with group A, there was a statistically significant difference (P<0.05) (Table 1) (Fig. 2).
Table 1.
Comparison of mean difference of PI and GI values for the three groups.
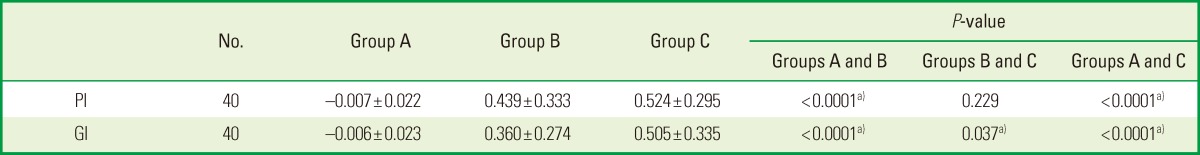
Values are presented as mean±standard deviation.
PI: plaque index, GI, gingival index, group A: patients were advised to rinse their mouths with 10 mL of distilled water for 1 minute twice daily for two weeks, group B: patients were advised to rinse their mouths with 0.2% chlorhexidine for 1 minute twice daily for two weeks, group C: patients were advised to rinse their mouths with triphala mouthwash for 1 minute twice daily for two weeks.
a)P<0.05 was considered significant.
Figure 2.
Bar diagram showing comparison of mean plague index (PI) and gingival index (GI) values for the three groups. Group A: patients were advised to rinse their mouths with 10 mL of distilled water for 1 minute twice daily for two weeks, group B: patients were advised to rinse their mouths with 0.2% chlorhexidine for 1 minute twice daily for two weeks, group C: patients were advised to rinse their mouths with triphala mouthwash for 1 minute twice daily for two weeks.
This result highlighted that statistically, there is no difference in the efficacy of CHX and triphala in terms of their antiplaque and anti-gingivitis properties. However, clinically, the mean difference in PI and GI was more for the triphala group than for the CHX group after 15 days of rinsing (Table 1).
Primary and secondary end points: within-group analysis
The intragroup analysis conducted using a Student paired t-test showed statistically significant results for the PI and GI values of group B and group C (P<0.05). There was no change in group A (Table 2).
Table 2.
Within-group comparison of plaque index and gingival index.
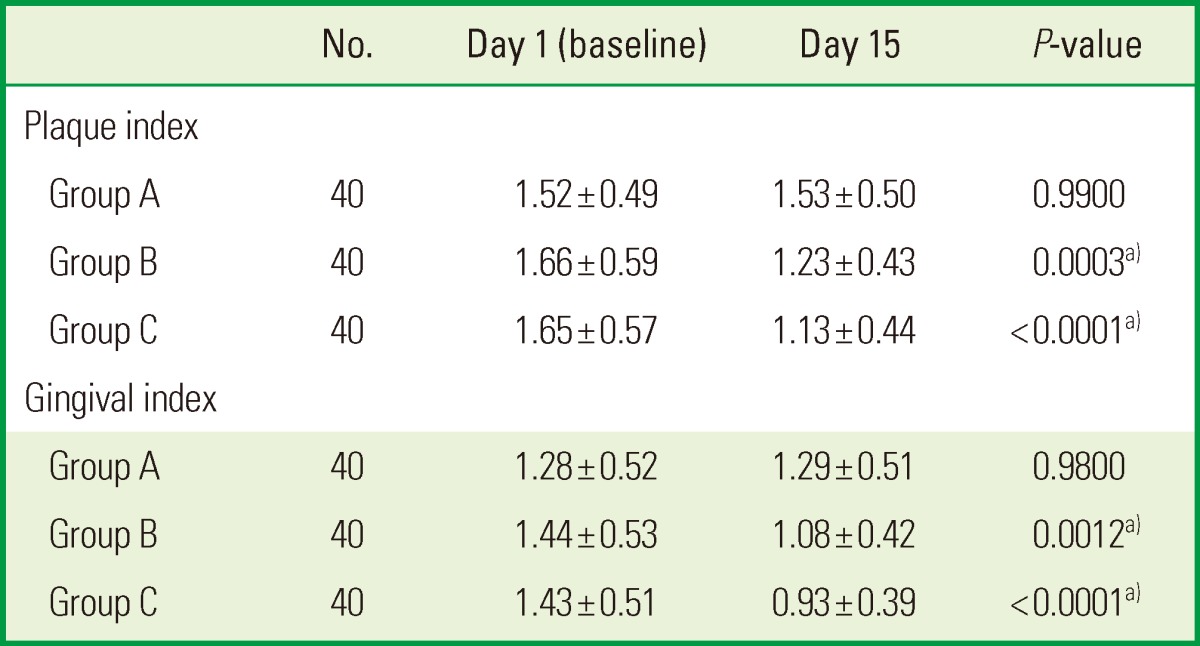
Values are presented as mean±standard deviation.
Group A: patients were advised to rinse their mouths with 10 mL of distilled water for 1 minute twice daily for two weeks, group B: patients were advised to rinse their mouths with 0.2% chlorhexidine for 1 minute twice daily for two weeks, group C: patients were advised to rinse their mouths with triphala mouthwash for 1 minute twice daily for two weeks.
a)P<0.05 was considered significant.
This signifies that there was a statistically and clinically significant change in GI and PI after 15 days in the triphala and the CHX groups (Fig. 2).
Side effects if any: within group
There were no side effects reported by patients in group A and group C. However, patients from group B reported that there was slightly altered taste sensation and burning sensation after rinsing with CHX.
DISCUSSION
Oral healthcare is an essential part of general health. Foreign bodies and bacteria in the mouth have been related to aspiration pneumonia and endocarditis [26]. Thus, in the midst of growing evidence of the connection between oral health and systemic health, herbal medicines with their "naturally occurring" active ingredients offer a gentle and enduring way for the restoration of health in the most trustworthy and least harmful way [21]. Since hospitalized, dependent, ill patients are more prone to oral diseases and discomfort, special oral care has to be taken in such patients [27]. Antiplaque and antimicrobial mouth rinses used thus far in periodontal practice contain either alcohol or sugar [28]. These ingredients enhance the cariogenic potential of the substrate and promote halitosis. Thus, by the use of an herbal mouth rinse, the inclusion of these ingredients and their obvious untoward effects may be avoided. This may improve oral hygiene [4].
This study was conducted in three different hospitals to compare the efficacy of an herbal mouthwash with CHX, which is considered to be the gold standard among antiplaque agents [4,5,7,8,9,10,11]. CHX is the most widely investigated and used oral product [6]. Short-term trials predominantly demonstrate the superior efficacy of CHX on plaque regrowth and numerous other outcome measures [11]. Plaque reductions of 16%-45% and gingivitis reduction from 27%-80% have been demonstrated in six-month trials [29]. Based on the accumulation of positive clinical research findings, CHX rinses are often used as a benchmark control, meaning a product already in use and/or evaluated, thus providing information regarding another agent's relative activity. CHX rinses are used similarly as a positive control, meaning that they are accepted as effective, the most effective, or the "gold standard" [5].
Triphala has been extensively used in Ayurveda because of its various properties and therapeutic uses. Triphala, meaning "three fruits" [17,24], an herb originating in India, has been found to act as a complete body cleanser. Not only does triphala help to detoxify and cleanse the colon, but it also purifies the blood and removes toxins from the liver. Other cleansing benefits of triphala include reduction of some forms of cholesterol (serum cholesterol) and of high blood pressure. Triphala can bring relief to a wide variety of stomach-related ailments such as abdominal pain, decreased appetite, stomach acidity, and constipation. For instance, this particular herb can be quite effective in treating common respiratory ailments such as cold and cough and has also been shown to be very beneficial for the reproductive system. It contains tannins, phenols, and glycosides, which are responsible for its strong antioxidant activity apart from its immunomodulatory, anti-inflammatory, analgesic, astringent, antispasmodic, anti-mutagenic, anticancer, and antimetastatic properties [20,30]. Triphala has been tested as an antioxidant and as a radioprotector in mice [16,20]. These attributes make triphala an effective remedy for geriatric degenerative diseases [30].
Several authors have used triphala as a mouth rinse in healthy gingivitis and periodontitis patients [23,31,32]. Triphala presented an antiplaque efficacy similar to that of CHX and was more effective in inhibiting plaque formation with lesser or no side effects [33]. Sushruta Samhita has emphasized that triphala has hemostatic, anti-inflammatory, analgesic, and wound-healing properties. Haritaki is the most efficacious for bleeding gums and gingival ulcers as well as carious teeth [17]. On the other hand, Amalaki contains a large amount of vitamin C, which is the most effective in preventing bleeding from gums [31]. The antimicrobial activity of triphala churna on a standard Streptococcus Mucous strain and a clinical isolate has been proved by the agar gel diffusion method [24]. Jagadish et al. [34] conducted a study to determine the effect of triphala on dental bio-films and concluded that triphala had potent antioxidant and antimicrobial activity and inhibited the growth of Streptococcus mutans and gram-positive cocci involved in plaque formation when it was adsorbed on the tooth surface. Tandon et al. [35] suggested the use of triphala mouthwash for preventing the development of incipient lesions and reported that triphala mouthwash is cheaper than the commercially available CHX mouthwash. Being an Ayurvedic product, it has no side effects and hence is safer for long-term use [35].
Thus, the present study was designed to evaluate triphala's anti-plaque and antigingivitis effects in dependent, ill, hospitalized patients who had untreated periodontal disease. The three hospitals were chosen from areas near the college campus. The patients having any periodontal disease according to the American Academy of Periodontology classification 1999, irrespective of their clinical attachment loss/probing pocket depth (CAL/PPD), with similar socioeconomic status and oral hygiene practice, in the age group of 20-65 years were chosen for the study. These patients were divided into three groups by block randomization. Group A (n=40) in Subdistrict Government Hospital, Kodoli, used distilled water; group B (n=40) in Mahatma Gandhi Hospital, New Pargaon, were provided with 0.2% CHX mouthwash, and group C (n=40), consisting of patients admitted in Yeshwant Chavan Hospital, was given triphala mouthwash. The division into groups was done in such a way that there was no intermingling of patients from different groups. This was done to avoid sample contamination by preventing any discussion amongst the patients on the type and taste of the mouthwash. The patients were advised to continue with their routine oral hygiene practices. They were instructed to rinse their mouth with 10 mL of the given solution for 1 minute twice daily for a period of two weeks. According to Eley [3], CHX should never be used for more than two weeks to avoid its local side effects such as teeth staining and taste alteration. Thus, the study period was limited to two weeks. The patients were instructed not to rinse their mouth with water or drink anything for 30 minutes after using the given mouth rinse. A similar amount and duration of mouthwash administration was followed in a study conducted by Axelsson and Lindhe [36]. A disclosing agent was used in all patients to determine the plaque levels as well as to motivate patients to maintain good oral hygiene. PI and GI were recorded in every patient on the first and the fifteenth day by different trained examiners to avoid bias.
Among 120 patients, 78 were males, and out of them, 19 were tobacco users. In this study, we did not isolate any patient based on his/her habits or medical history because the antiplaque agents that we used are not contra-indicated in any patients except for patients allergic to CHX. Triphala is gentle for people of all ages, from children to seniors and hence, is recommended for everybody. Triphala is beneficial and safe even if ingested by hospitalized ill patients [2]. Since this study was conducted in medical hospitals, scaling and root planing were not performed during the time of study. The authors did not expect any changes in CAL/PPD only upon rinsing with the mouth rinse. Therefore, only GI and PI were evaluated to check the efficacy of mouth rinses on gingival inflammation.
In this study, PI and GI showed a statistically significant difference in group B and group C after 15 days (P<0.05). This signifies that both the agents used in the study are efficient antiplaque and antigingivitis agents. Our study was in accordance with results obtained by Desai et al. [23], Maurya et al. [31], and Bajaj and Tandon [32]. Triphala as a mouthwash showed significant reduction in periodontal indices when compared to scaling and root planing alone, but no significant difference was noted between the triphala and the CHX groups [23]. In our clinical trial, when group B was compared to group C, there was no statistically significant difference (P>0.05). This signified that the efficacy of triphala mouthwash was similar to that of 0.2% CHX. Our results were corroborated by Bajaj and Tandon [32], in their nine-month study. Thus, based on these findings, triphala can be considered the best alternative to CHX. Some patients in the CHX group complained about metallic taste and slight soreness in the mouth after use. In the triphala group, patients did not complain about any side effects. Rather, patients gave good feedback for the use of this herbal mouthwash as it had no adverse effects and was readily available at affordable prices in this geographical location for future use.
As microbial plaque is one of the risk factors of periodontal disease, every effort has to be taken for the prevention of its formation and accumulation. Thus far, CHX has been considered the best antiplaque and antigingivitis agent, but now, it is time to acknowledge the value of natural herbs like triphala, known to have many useful properties and no side effects. More studies are required to further emphasize the effect of triphala on gram-negative anaerobes, the microorganisms responsible for causing periodontitis, and to determine the sustained release capacity (substantivity) of triphala for plaque control for the prevention of periodontal disease and maintenance of good oral health.
In conclusion, this trial highlights that triphala (herbal) mouthwash is as efficient an antiplaque agent as 0.2% CHX. It is significantly useful in reducing plaque accumulation and gingival inflammation, thereby preventing periodontal disease in the studied patients. It is also cost effective, easily available, and well tolerated with no reported side effects as compared to the gold standard, CHX.
ACKNOWLEDGEMENTS
The authors sincerely thank the staff and the Principal of Yeshwant Chavan Ayurveda College, Kodoli, Kolhapur, Maharashtra, for their constant help and support during the study.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 2.Axelsson P. In: Lang N, Karring T, editors. Mechanical plaque control; Proceedings of the 1st European workshop on periodontics, 1993; London: Quitessence; 1994. pp. 219–243. [Google Scholar]
- 3.Eley BM. Antibacterial agents in the control of supragingival plaque: a review. Br Dent J. 1999;186:286–296. doi: 10.1038/sj.bdj.4800090. [DOI] [PubMed] [Google Scholar]
- 4.Nagappan N, John J. Antimicrobial efficacy of herbal and chlorhexidine mouth rinse: a systematic review. J Dent Med Sci. 2012;2:5–10. [Google Scholar]
- 5.Addy M, Moran JM. Evaluation of oral hygiene products: science is true; don't be misled by the facts. Periodontol 2000. 1997;15:40–51. doi: 10.1111/j.1600-0757.1997.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 6.Asadoorian J. Oral rinsing. Can J Dent Hyg. 2006;40:1–13. [Google Scholar]
- 7.Loe H, Schiott CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970;5:79–83. doi: 10.1111/j.1600-0765.1970.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 8.Imfeld T. Chlorhexidine-containing chewing gum. Clinical documentation. Schweiz Monatsschr Zahnmed. 2006;116:476–483. [PubMed] [Google Scholar]
- 9.Van Leeuwen MP, Slot DE, Van der. Essential oils compared to chlorhexidine with respect to plaque and parameters of gingival inflammation: a systematic review. J Periodontol. 2011;82:174–194. doi: 10.1902/jop.2010.100266. [DOI] [PubMed] [Google Scholar]
- 10.Rolla G, Loe H, Schiott CR. Retention of chlorhexidine in the human oral cavity. Arch Oral Biol. 1971;16:1109–1116. doi: 10.1016/0003-9969(71)90215-9. [DOI] [PubMed] [Google Scholar]
- 11.Adams D, Addy M. Mouthrinses. Adv Dent Res. 1994;8:291–301. doi: 10.1177/08959374940080022401. [DOI] [PubMed] [Google Scholar]
- 12.Flotra L, Gjermo P, Rolla G, Waerhaug J. Side effects of chlorhexidine mouth washes. Scand J Dent Res. 1971;79:119–125. doi: 10.1111/j.1600-0722.1971.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 13.Overholser CD., Jr Longitudinal clinical studies with antimicrobial mouthrinses. J Clin Periodontol. 1988;15:517–519. doi: 10.1111/j.1600-051x.1988.tb01024.x. [DOI] [PubMed] [Google Scholar]
- 14.Addy M. Chlorhexidine compared with other locally delivered antimicrobials: a short review. J Clin Periodontol. 1986;13:957–964. doi: 10.1111/j.1600-051x.1986.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 15.Bose S, Sinha SK, Mukherjee G. In-vitro study of triphala on antioxidant activity. Sci Cult. 2011;77:511–513. [Google Scholar]
- 16.Surya Prakash DV, Sree Satya N, Avanigadda S, Vangalapati M. Pharmacological review on Terminalia chebula. Int J Res Pharm Biomed Sci. 2012;3:679–683. [Google Scholar]
- 17.Dar PA, Sofi G, Parray SA, Jafri MA. Halelah siyah (Terminalia chebula retz): in unani system of medicine and modern pharmacology: a review. Int J Inst Pharm Life Sci. 2012;2:138–149. [Google Scholar]
- 18.Vani T, Rajani M, Sarkar S, Shishoo CJ. Antioxidant properties of the Ayurvedic formulation Triphala and its constituents. Pharm Biol. 1997;35:313–317. [Google Scholar]
- 19.Padmawar A, Bhadoriya U. Phytochemical investigation and comparative evaluation of in vitro free radical scavenging activity of Triphala & Curcumin. Asian J Pharm Med Sci. 2011;1:9–12. [Google Scholar]
- 20.Ariyaphong W, Kanjana J, Seewaboon S. Triphala: The Thai traditional herbal formulation for cancer treatment. Songklanakarin J Sci Technol. 2009;31:139–149. [Google Scholar]
- 21.Malhotra R, Grover V, Kapoor A, Saxena D. Comparison of the effectiveness of a commercially available herbal mouthrinse with chlorhexidine gluconate at the clinical and patient level. J Indian Soc Periodontol. 2011;15:349–352. doi: 10.4103/0972-124X.92567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham S, Kumar MS, Sehgal PK, Nitish S, Jayakumar ND. Evaluation of the inhibitory effect of triphala on PMN-type matrix metalloproteinase (MMP-9) J Periodontol. 2005;76:497–502. doi: 10.1902/jop.2005.76.4.497. [DOI] [PubMed] [Google Scholar]
- 23.Desai A, Anil M, Debnath S. A clinical trial to evaluate the effects of triphala as a mouthwash in comparison with chlorhexidine in chronic generalized periodontitis patient. Indian J Dent Adv. 2010;2:243–247. [Google Scholar]
- 24.Thomas B, Shetty SY, Vasudeva A, Shetty V. Comparative evaluation of antimicrobial activity of triphala and commercially available toothpastes: an in-vitro study. Int J Public Health Dent. 2011;2:8–12. [Google Scholar]
- 25.Biradar YS, Jagatap S, Khandelwal KR, Singhania SS. Exploring of antimicrobial activity of triphala Mashi-an Ayurvedic formulation. Evid Based Complement Alternat Med. 2008;5:107–113. doi: 10.1093/ecam/nem002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffiths J, Jones V, Leeman I, Lewis D, Patel K, Wilson K. Guidelines for the development of local standards of oral health care for dependent, dysphagic, critically and terminally ill patients. Revised. [London]: British Society for Disability and Oral Health; 2000. [Google Scholar]
- 27.Sumi Y, Nakamura Y, Nagaosa S, Michiwaki Y, Nagaya M. Attitudes to oral care among caregivers in Japanese nursing homes. Gerodontology. 2001;18:2–6. doi: 10.1111/j.1741-2358.2001.00002.x. [DOI] [PubMed] [Google Scholar]
- 28.Leyes Borrajo JL, Garcia VL, Lopez CG, Rodriguez-Nunez I, Garcia FM, Gallas TM. Efficacy of chlorhexidine mouthrinses with and without alcohol: a clinical study. J Periodontol. 2002;73:317–321. doi: 10.1902/jop.2002.73.3.317. [DOI] [PubMed] [Google Scholar]
- 29.Santos A. Evidence-based control of plaque and gingivitis. J Clin Periodontol. 2003;30(Suppl 5):13–16. doi: 10.1034/j.1600-051x.30.s5.5.x. [DOI] [PubMed] [Google Scholar]
- 30.Gupta M. Therapeutic uses of the polyherbal drug triphala in geriatric diseases. Int J Pharm Bio Sci. 2010;1:1–13. [Google Scholar]
- 31.Maurya DK, Mittal N, Sharma KR, Nath G. Role of triphala in the management of peridontal disease. Anc Sci Life. 1997;17:120–127. [PMC free article] [PubMed] [Google Scholar]
- 32.Bajaj N, Tandon S. The effect of triphala and chlorhexidine mouthwash on dental plaque, gingival inflammation, and microbial growth. Int J Ayurveda Res. 2011;2:29–36. doi: 10.4103/0974-7788.83188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narayan A, Mendon C. Comparing the effect of different mouthrinses on de novo plaque formation. J Contemp Dent Pract. 2012;13:460–463. doi: 10.5005/jp-journals-10024-1169. [DOI] [PubMed] [Google Scholar]
- 34.Jagadish L, Anand Kumar VK, Kaviyarasan V. Effect of triphala on dental bio-film. Indian J Sci Technol. 2009;2:30–33. [Google Scholar]
- 35.Tandon S, Gupta K, Rao S, Malagi KJ. Effect of triphala mouthwash on the caries status. Int J Ayurveda Res. 2010;1:93–99. doi: 10.4103/0974-7788.64413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Axelsson P, Lindhe J. Efficacy of mouthrinses in inhibiting dental plaque and gingivitis in man. J Clin Periodontol. 1987;14:205–212. doi: 10.1111/j.1600-051x.1987.tb00968.x. [DOI] [PubMed] [Google Scholar]