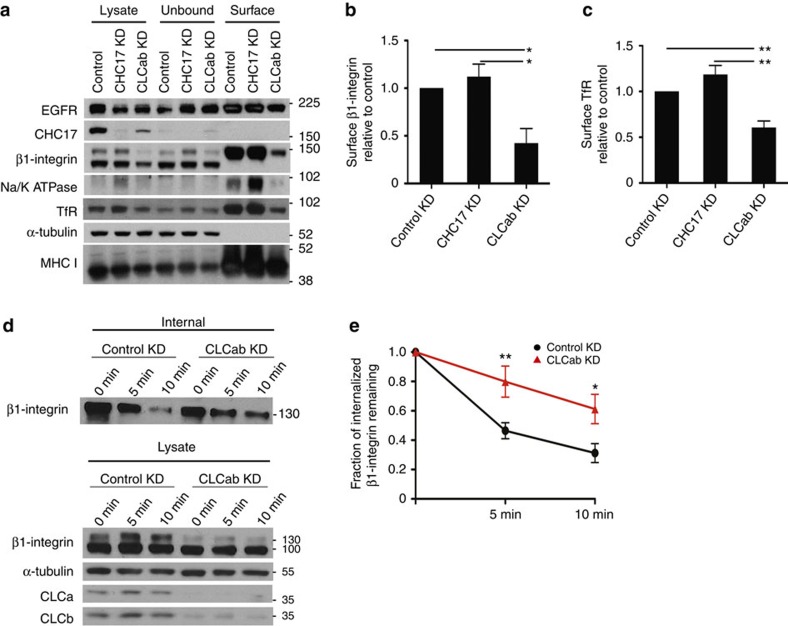
Figure 4. CLC depletion reduces constitutive recycling of β1-integrin and steady state levels of other surface molecules.
(a) HeLa cells transfected with the indicated siRNAs for knockdown (KD) were biotinylated for 30 min and surface biotinylated proteins were isolated using streptavidin beads, followed by immunoblotting for the indicated proteins. (b,c) Quantification of surface levels of (b) β1-integrin and (c) transferrin receptor (TfR) from a and replicate experiments (mean±s.e.m.; n=3; *P<0.05, **P<0.01, one-way analysis of variance (ANOVA) followed by Newman–Keuls post hoc test). (d) Recycling of internalized β1-integrin in control and CLCab-siRNA-treated HeLa cells. Cells were biotinylated and allowed to internalize surface proteins for 30 min at 37 °C. Surface biotin was removed by reduction and internalized proteins were chased back to the cell surface for the indicated times at 37 °C. Surface biotin was reduced again, and cells were lysed. Above: Biotinylated proteins were bound to streptavidin beads, followed by immunoblotting for β1-integrin. Below: Immunoblots of indicated proteins in total cell lysate at each time point. Representative immunoblots of one experiment (n=6). (e) Quantification of β1-integrin recycling assays as in d (mean±s.e.m.; n=6; *P<0.05, **P<0.01, two-way ANOVA followed by Bonferroni post hoc test). Migration positions of molecular mass markers are indicated in kDa at the right of the immunoblots shown.