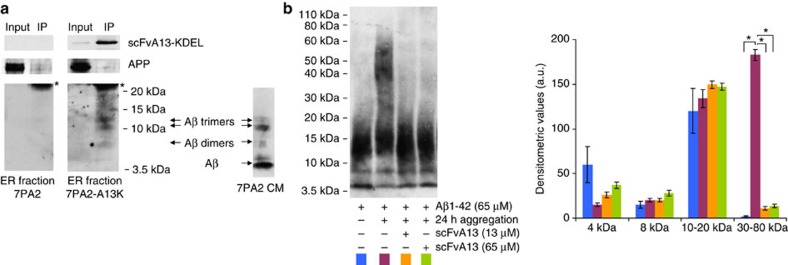
Figure 7. Intrabody binding of AβOs in the ER and interference of scFvA13 with Aβ assembly in vitro.
(a) Intrabody-mediated selective pulldown of AβOs: co-IP of scFvA13-KDEL intrabody with Aβ dimers, Aβ trimers and larger AβOs (<20 kDa) but not with Aβ monomers nor APP, from an ER-enriched fraction of 7PA2-A13K cells (7PA2 cells as intrabody negative control). The co-IP was obtained by an anti-V5 coupled resin, to bind the C-terminal V5 tag of the intrabody and blotted to detect the different Aβ species and APP (mAb 6E10) and for the intrabody (anti-V5). * indicates the light chain of the anti-V5 IP antibody. On the right, representative Aβ bands detected (by 6E10-WB) in 7PA2 CM in the 3.5–15 kDa range. (b) WB analysis (rabbit mAb anti-Aβ) of Aβ assembled in vitro with or without scFvA13. The human Aβ1-42 peptide (65 μM) was aggregated at 22 °C for 24 h without scFvA13, or in the presence of substoichiometric (13 μM, ratio Aβ:scFv, 1:5) or stoichiometric concentrations (65 μM) of scFvA13. According to previous reports47, after the in vitro assembly, synthetic Aβ1-42 shows a pattern of HMW oligomers (30–80 kDa) absent at the early steps of aggregation (in which Aβ monomers and LMW oligomers (<20 kDa) prevail). Of note, the co-presence of LMW oligomers and monomers cannot be ruled out in starting solutions of synthetic Aβ70, being these species in rapid equilibrium and determining variability on the levels of Aβ monomers measured. Both sub- and stoichiometric concentrations of scFvA13 during the assembly of Aβ1-42 determine the following: (i) the lack of formation of HMW AβOs; (ii) a pattern of LMW AβOs similar to that at the early steps of aggregation. This shows that the scFvA13 interferes with critical conformational changes during the early assembly, avoiding the formation of larger AβOs. The histogram shows the variations of Aβ species obtained from densitometric analysis. Mean values±s.e.m., n=3; *P<0.01 (Student’s t-test).