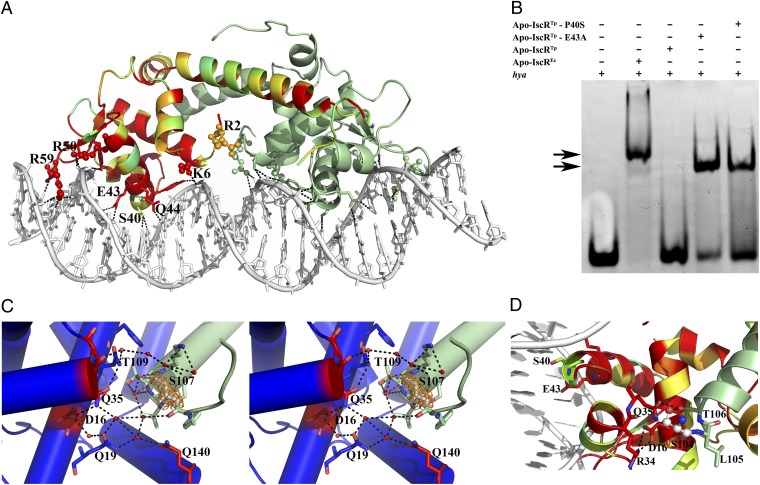
Fig. 4.
Binding of IscR to type-2 promoter sequences. (A) Bidentate binding of apo-IscREc (C92/98/104S) to the hya promoter DNA sequence. In one of the monomers, residues are colored according to conservation, where red corresponds to positions strictly conserved between E. coli and T. potens IscR. Residues at the DNA-interacting interface are highlighted as sticks and basic residues as spheres. Hydrogen bonds between apo-IscREc and DNA are represented as dotted lines. (B) Electrophoretic mobility-shift assay analysis of apo-IscR binding to the E. coli hya promoter. Arrows denote observed band-shifts. (C) Stereoscopic view of the intricate network of hydrogen bonds in apo-IscRTp (one monomer of the functional dimer is colored green and the other one blue) centered on the cluster-binding residue 107 (light gray). The 2Fo − Fc electron density map around residue 107 is represented as an orange mesh. Water molecules and strictly conserved residues in closely related IscR molecules are colored red. (D) In apo-IscREc (C92/98/104S), serine 104 (ball and stick) participates in a network of polar interactions with neighboring residues (sticks), cross-linking helices α1, α2, and α5. The corresponding cysteine residue in the wild-type protein could be part of a sensing mechanism for the presence of the Fe/S cluster.