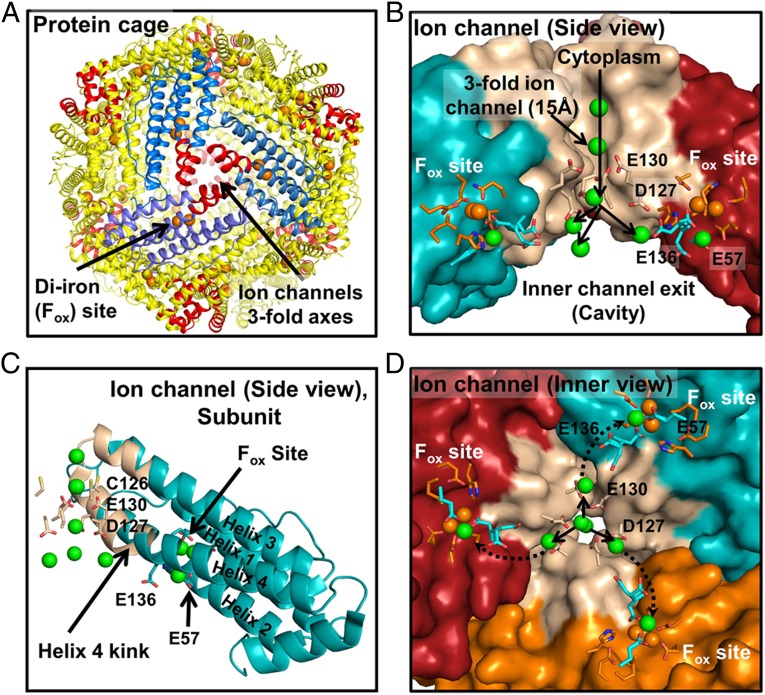
Fig. 1.
Ion channels in soluble ferritin protein nanocages transport Fe2+ to multiple catalytic centers (Fox or oxidoreductase sites). (A) The 24-subunit ferritin protein cage. Red helices, Fe2+ channels. (B) A ferritin Fe2+ ion channel (side view). Green spheres, metal ions. Conserved residues E57 and E136 are putative carboxylate links in Fe2+ transit between ferritin ion channel carboxylate (E130 and D127) and ferritin enzyme (Fox) sites based on phylogenetic structural location and conformational flexibility in protein crystals (16, 18). (C) A single ferritin protein cage subunit (side view). Tan, channel helix segments. (D) Ion channel (viewed from inside the protein cage). Green spheres, metal ions. Prepared from PDB 3KA3 using PyMol.