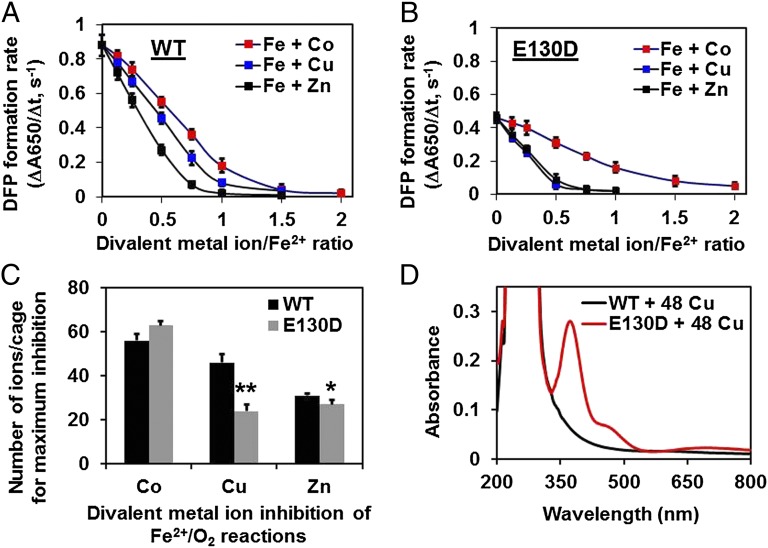
Fig. 3.
Selective effects of Co2+, Cu2+, and Zn2+ on ferritin activity. Solutions of metal cation inhibitors Co2+, Cu2+, or Zn2+ (6–96 metal ions/protein cage) were added to ferritin protein cage solutions in 0.2 M Mops⋅Na, 0.2 M NaCl, pH 7.0, for 1 h, before rapidly mixing (<5 ms) with acidic solutions of FeSO4 (48 Fe2+/cage) at 25 °C; final protein concentrations were 50 µM, subunits (2.04 µM, protein cages). (A and B) Effects of Co2+, Cu2+, and Zn2+ on 2Fe2+/O2 catalysis (diferric peroxo, A650 nm, formation): (A) WT and (B) E130D. (C) Comparisons of metal cation inhibition in WT and E130D ferritin at 48 Fe/cage (2 Fe/enzyme site). Data for ferritin E130D with Cu2+ and Zn2+ were significantly different from WT: *P < 0.01; **P < 0.001. The inhibition of enzyme activity by metal cations in A of ferritins E136A and E57A/E136A was so great that reliable measurements were not possible. (D) Differences in UV-vis spectrum of Cu2+-WT ferritin and Cu2+-E130D ferritin. The spectrum of Cu2+-E130D ferritin is of a Cu–Cys charge transfer complex. The data in A–C are averages (±SD). All data are from two to four independent experiments, using two to three different protein preparations of each protein.