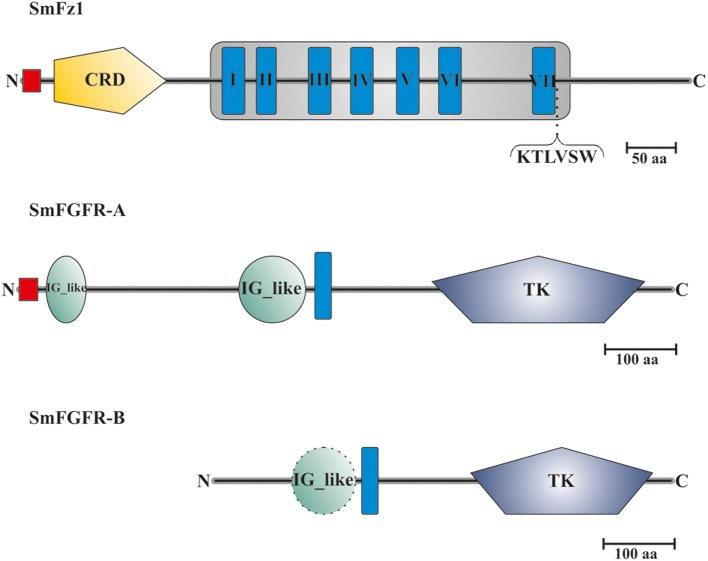
Figure 2.
Domain structure analyses of SmFz1, SmFGFR-A, and SmFGFR-B. Schematic structures of the frizzled homolog SmFz1, and the two FGFR homologs SmFGFR-A and SmFGFR-B from S. mansoni. SmFz1 comprises an N-terminal signal peptide sequence (red) and an extracellular CRD domain (yellow) sufficient for Wnt ligand binding. The frizzled transmembrane domain (gray box) contains seven TMHs (I–VII). Following TMH VII, a Dvl-binding motif (KTLVSW) is located at the beginning of the intracellular C-terminus. Both FGFRs possess TK domains within their intracellular C-termini but differ in the structures of their extracellular parts (TMHs in blue). SmFGFR-A consists of an N-terminal signal peptide sequence (red) followed by two IG-like domains (green) sufficient for ligand binding (A). In contrast the N-terminus of SmFGFR-B is smaller in size and contains only one putative IG-like domain (dashed line), which was not rated as significant (B).