Abstract
Pancreaticoduodenectomy (PD) specimens present a challenge for surgical pathologists because of the relative rarity of these specimens, combined with the anatomic complexity. Here, we describe our experience on the orientation, dissection, and sampling of PD specimens for a more practical and accurate evaluation of pancreatic, distal common bile duct (CBD), and ampullary tumors. For orientation of PDs, identification of the “trapezoid,” created by the vascular bed at the center, the pancreatic neck margin on the left, and the uncinate margin on the right, is of outmost importance in finding all the pertinent margins of the specimen including the CBD, which is located at the upper right edge of this trapezoid. After orientation, all the margins can be sampled. We submit the uncinate margin entirely as a perpendicular inked margin because this adipose tissue–rich area often reveals subtle satellite carcinomas that are grossly invisible, and, with this approach, the number of R1 resections has doubled in our experience. Then, to ensure proper identification of all lymph nodes (LNs), we utilize the orange-peeling approach, in which the soft tissue surrounding the pancreatic head is shaved off in 7 arbitrarily defined regions, which also serve as shaved samples of the so-called “peripancreatic soft tissue” that defines pT3 in the current American Joint Committee on Cancer TNM. With this approach, our LN count increased from 6 to 14 and LN positivity rate from 50% to 73%. In addition, in 90% of pancreatic ductal adenocarcinomas there are grossly undetected microfoci of carcinoma. For determination of the primary site and the extent of the tumor, we believe bisectioning of the pancreatic head, instead of axial (transverse) slicing, is the most revealing approach. In addition, documentation of the findings in the duodenal surface of the ampulla is crucial for ampullary carcinomas and their recent site-specific categorization into 4 categories. Therefore, we probe both the CBD and the pancreatic duct from distal to the ampulla and cut the pancreatic head to the ampulla at a plane that goes through both ducts. Then, we sample the bisected pancreatic head depending on the findings of the case. For example, for proper staging of ampullary carcinomas, it is imperative to take the sections perpendicular to the duodenal serosa at the “groove” area, as ampullary carcinomas often extend to this region. Amputative (axial) sectioning of the ampulla, although good for documentation of the peri-Oddi spread of the intra-ampullary tumors, unfortunately disallows documentation of mucosal spread of the papilla of Vater tumors (those arising from the edge of the ampulla, where the ducts transition to duodenal mucosa and extending) into the neighboring duodenum. Axial sectioning also often fails to document tumor spread to the “groove” area. In conclusion, knowledge of the gross characteristics of the anatomic hallmarks is essential for proper dissection of PD specimens. The approach described above allows practical and accurate documentation and staging of pancreas, distal CBD, and ampullary cancers.
Keywords: pancreas, ampulla, common bile duct, grossing, pancreaticoduodenectomy, Whipple
Pancreaticoduodenectomies (PDs) are challenging specimens, mostly because of the anatomic complexity of the region, where various structures come together, combined with lack of familiarity, owing to the rarity of the operation. Until recently, PD was rarely performed because of its high mortality rate. With the improvements in surgical techniques and perioperative care in the past 2 decades, the mortality rate of PD has now reduced to <2% in experienced hands.1,2 Meanwhile, the advancements of imaging technology have made the pancreas infinitely more accessible. Thus, PD is now performed routinely not only in major medical centers but also in many smaller hospitals. Consequently, pancreatic pathology, including “incidentalomas,”3,4 have begun to come to clinical attention and to the surgical pathologists more commonly in daily practice.5
Different approaches have been put forth for the pathologic examination of pancreatic specimens.6–13 In this article, we document our experience on the basis of 1080 PDs personally dissected and examined at Wayne State (1997 to 2006) and Emory (2007 to 2012) Universities.
PANCREATICODUODENECTOMY OR PANCREATICOGASTRODUODENECTOMY OR “WHIPPLE” CONTENTS OF THE SPECIMEN
At a bare minimum, a PD specimen contains the pancreatic head, most of the duodenum, and the distal segment of the common bile duct (CBD). If a “classic” Whipple procedure is used, it also contains the pylorus and a segment of the stomach antrum. This has become less common in favor of the pylorus-preserving option, unless oncologic circumstances necessitate the partial gastrectomy or the patient has received preoperative radiation. In addition, the gallbladder and cystic duct are removed routinely. Briefly, for the purposes of surgical pathologists to know and understand, the operation involves the following steps:
-
(1)
The pancreatic neck is transected from the body of the pancreas at the region of the major vessels, normally directly anterior to the superior mesenteric/portal vein. The remaining pancreas (body and tail) with its duct is then sewed to the jejunum or the stomach, depending on the reconstruction chosen.
-
(2)
The pancreatic head is dissected from the retroperitoneal soft tissues, where it is embedded and loosely and imperceptibly blends with the retroperitoneal adipose tissue. In the posterior-inferior aspect of the uncinate process, the pancreatic tissue is dissected from the retroperitoneal soft tissues, just right lateral along the superior mesenteric artery. This constitutes the uncinate (retroperitoneal) margin (see below).
-
(3)
The pancreatic head is also freed from the superior mesenteric/portal vein and superior mesenteric artery, all of which form a groove (indentation) on the posterior-inferior aspect of the pancreas that we refer to as the vascular bed.
-
(4)
The CBD is cut at the level of the distal third-to-half before it enters into the pancreas, and its distal (intrapancreatic) segment becomes a part of the specimen, whereas the remaining proximal segment is anastomosed to the jejunum.
-
(5)
For a pylorus-preserving operation, the duodenum is transected approximately 1 to 2 cm distal to the pylorus. For a classic Whipple, the antrum is transected, thus including the distal stomach and pylorus in the specimen. For both versions of the PD, the proximal jejunum is transected approximately 5 to 10 cm distal to the ligament of Treitz. Thus, for a pylorus-preserving operation, 1 to 2 cm of the duodenum is preserved, whereas the entire duodenum is removed during a classic Whipple procedure.
“Extended” PD, which involves dissection of retroperitoneal and aorta-caval lymph nodes (LNs), is a highly demanding operation with dubious oncologic benefits and is seldom used these days, with the exception of a few institutions in Asia.14 The lack of interest in this operation in the West appears to be based on the belief that including the retroperitoneal LNs, in an effort to make the operation more oncologically complete, does not translate to improved patient survival and is associated with increased complications and morbidity.15,16
TERMINOLOGY ISSUES
Terminology of pancreatic resection margins is confusing as different terms have been used in publications to describe these margins (Table 1). For instance, the region where the pancreatic neck is transected and the head is surgically separated from the rest of the pancreas (Figs. 1, 2) has been termed variably as pancreatic neck margin, pancreatic duct margin, and (distal) pancreatic resection margin. We believe that the term “pancreatic resection margin” may be mistaken by surgeons and radiation oncologists, as other margins of a PD specimen are also technically resection margins. Thus, we prefer to refer to this margin as the “pancreatic neck margin” to be more specific and distinguish it from other resection margins.
TABLE 1.
Structures on the Surfaces of a PD
| Term in This Study | Synonyms | Reporting Recommendation (Authors' Approach) | Reporting Recommendation (American Joint Committee on Cancer17) | Reporting Recommendation (College of American Pathologists, as of October 1, 201318) | Reporting Recommendation (Royal College Pathologists19 and Verbeke et al11) | Reporting Recommendation (Esposito et al,20 Schlitter and Esposito21 and Maksymov et al13) |
|---|---|---|---|---|---|---|
| CBD margin | Margin | Margin | Margin | Margin | Margin | |
| Pancreatic neck margin (Figs. 1, 2) | Pancreatic duct margin | Margin | Margin | Margin | Margin | Margin |
| Distal resection margin | ||||||
| Uncinate margin (Figs. 1, 2, 5) | Superior mesenteric artery margin | Margin | Margin | Margin | Margin | Margin |
| Retroperitoneal margin | ||||||
| Interior-posterior margin | ||||||
| Interior-posterior retroperitoneal margin | ||||||
| Mesopancreatic margin | ||||||
| Medial margin | ||||||
| Radial margin | ||||||
| Vascular bed (Figs. 1, 2) | Vascular groove | Surface* | – | Surface† (Part of the deep retroperitoneal posterior surface) | Margin | Margin‡ |
| Superior mesenteric/portal vein margin | ||||||
| Anterior free surface (Fig. 3) | Anterior margin | Surface | – | – | Margin | Margin§ |
| Posterior free surface (Figs. 3, 9) | (Part of) uncinate margin | Surface | Posterior pancreatic margin (NOT the retroperitoneal margin) | Surface† | Margin | Margin |
| (Part of) retroperitoneal margin | ||||||
| Deep retroperitoneal posterior surface | ||||||
| Inferior vena cava margin |
The authors ink and take 1 perpendicular section from the vascular bed with the closest tumor and report it as vascular bed surface.
The American Joint Committee on Cancer and College of American Pathologists protocols recommend inking the posterior (free) surface of the pancreas including the vascular groove (bed) and submission of sections through the tumor at its closest approach to this surface.
It is regarded as a part of the medial (uncinate) margin by Esposito et al,20 Schlitter and Esposito.21
In Maksymov et al's13 opinion, the anterior margin is present in a pylorus-preserving PD and absent in a standard Whipple procedure.
FIGURE 1.
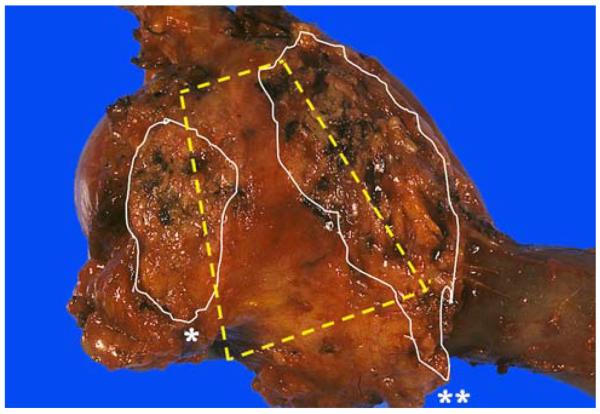
Laying the duodenum with the pancreas on top allows readily the identification of the “trapezoid,” located in the postero-median aspect of the pancreatic head. The left vertical edge of the trapezoid is formed by the pancreatic neck margin* (often cauterized, relatively flat and reveals fine granularities) and the right vertical edge by the uncinate margin** (elongated, relatively soft and convex with highly irregular/nodular appearance). A concave-shaped, smooth-surfaced, relatively firm area in between these 2 margins is the vascular bed, where the superior mesenteric vein/portal vein and superior mesenteric artery lie originally.
FIGURE 2.
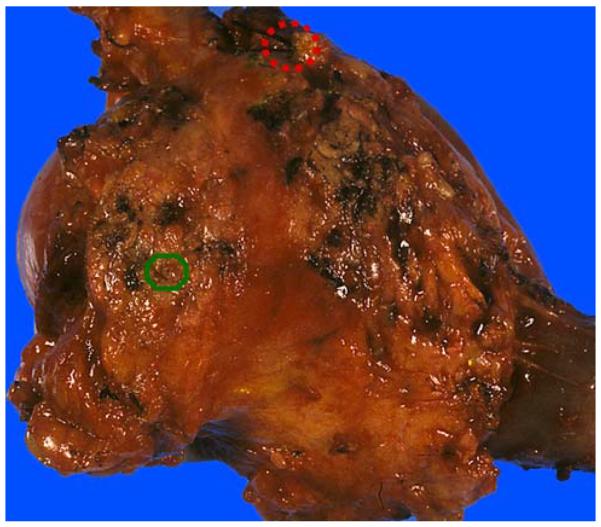
The pancreatic duct orifice (green circle), usually located in the upper right quadrant of the pancreatic neck, may be difficult to identify because of its small size. The CBD orifice (red dashed circle) is located at the plateau at the upper right edge of the uncinate margin. It is much larger than the pancreatic duct and should be identifiable in every case. If it has been stitched surgically, the removal of the stitch would make it readily accessible.
Similarly, for the area of the uncinate that is surgically dissected from the posterior retroperitoneal soft tissues (Figs. 1, 2), various terms including uncinate margin, retroperitoneal margin, posterior-inferior margin, deep margin, radial margin, mesopancreatic margin, superior mesenteric artery margin, and many more have been used. To be uniform and to avoid distraction; we will use the term “uncinate margin” throughout the manuscript.
More importantly, defining the uncinate margin and establishing whether it extends to encompass the entire posterior, including the serosa-covered pancreato-duodenal aspect of the specimen (Fig. 3) where the postero-lateral pancreatic head is readily peeled off from the tissues corresponding to the anterior surface of the inferior vana cava, are also controversial.9,10,13 Even the American Joint Committee on Cancer17 and the College of American Pathologists guidelines18 appear to disagree on the definition of this margin. Our approach to these structures (Table 1) in determining whether to designate them as margin or free surfaces is based on the oncologic pathology principles used in other organs such as the rectum, in which manually dissected (by surgeon) compartments are regarded as “margin,” and those that come off readily and are serosa covered are regarded as “free surfaces.” Accordingly, we currently prefer to refer to only the posterior-inferior aspect of the uncinate process as margin and to the posterior-right aspect of the pancreatic head as free surface. We also believe it is important to be uniform in using these principles. For example, for practical and biological purposes, the posterior-right aspect of the pancreatic head surface, which is covered by serosa in the region where it transitions into the duodenum (Fig. 3), is not exactly any different than the anterior aspect. Considering that the anterior aspect is almost uniformly referred to as free surface (ie, not a margin), we conclude that it would be appropriate to regard this postero-duodenal region as a surface as well. In daily practice, more important is to document these regions separately and clarify in a comment which margin or surface is being referred to.
FIGURE 3.
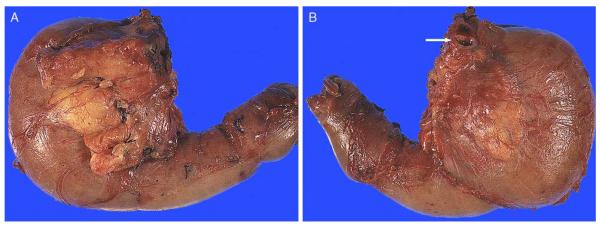
(A) Anterior surface. Anterior surface of a PD typically contains abundant adipose tissue and is convex in appearance. Transition of the pancreatic head to the duodenal wall is also often more irregular, showing a palpable sharp edge due to the overriding pancreatic parenchyma. (B) Posterior surface. In contrast with the anterior surface, the posterior surface is relatively flat, smooth, and glistening. Transition from the pancreatic head to the duodenal wall (pancreaticoduodenal junction) is fairly smooth as well, with the pancreaticoduodenal “groove” only barely identifiable. Note the CBD orifice at the superior edge, at the plateau (arrow).
Along the same lines, we believe it is more appropriate to document the vascular bed (where the portal vein and the mesenteric vein form a groove in the head, adjacent to the uncinate process) (Figs. 1, 2) separately from the uncinate process margin, as it is composed of compact pancreatic tissue that is demarcated and is readily peeled off (rather than manually dissected) from these large vessels.
ORIENTATION AND IDENTIFICATION OF IMPORTANT STRUCTURES IN THE GROSS ROOM
We have found, by working with our residents and pathologists' assistants over the years, that the following sets of instructions are the most practical toward proper orientation of the specimen and identification of the pertinent structures.
-
(1)
Identify the “trapezoid”: When the duodenum is laid on the table and stretched and the pancreas is suspended above it by the prosector, a distinctive region of the pancreas that we refer to as the “trapezoid” in the postero-median aspect of the pancreatic head is exposed (Fig. 1). Center of the trapezoid is a concave-shaped, smooth-surfaced, relatively firm area corresponding to the groove in which the superior mesenteric/portal vein lies originally (vascular bed, Fig. 1). The left vertical edge of the trapezoid is formed by the pancreatic neck margin and the right vertical edge by the uncinate margin.
-
(2)Localize the 3 most pertinent structures: Once the trapezoid has been properly identified, the 3 most pertinent margins, the trio—pancreatic neck margin, uncinate margin, and CBD margin—can be recognized easily.
- Pancreatic neck margin: The pancreatic neck margin, which is often cauterized, is ovoid, relatively flat, and shows fine granularities of the dense pancreatic tissue (not appreciated at other surfaces). Looking at this region directly, the pancreatic duct orifice is usually located in the upper outer (right) quadrant and on occasion may be very close to the edge. The orifice is often difficult to identify by visual inspection because it is narrower than 2 to 3 mm (Fig. 2). Squeezing of the pancreatic head sometimes leads to the backwash release of a drop or 2 of the pancreatic juice from the orifice (if the pancreas had not been manipulated extensively beforehand), which may be very helpful. However, this test can be performed only once or twice because usually there is minimal amount of juice in the duct that is released easily by the earlier manipulations, and that is why it is advisable to prepare for this test by cleaning this area and observing the target region closely while squeezing the pancreatic head toward this region. Moreover, if the pancreatic duct orifice cannot be visualized, a thin shave of the cauterized edges of this area (in which artefacts play a role in hiding the orifice) may help. Once the edge is removed, the orifice usually becomes more visible, as a white, thin-walled punctuate structure, standing out from the rest of the dense pancreatic lobular tissue.
- Uncinate margin: The uncinate margin constitutes the right vertical edge (postero-lateral) of the trapezoid. It can be recognized as an elongated, relatively soft, and convex area with a highly “lumpy-bumpy” appearance, which is in stark contrast with the vascular bed. This “lumpy-bumpy” appearance reflects not only the varie-gated nature of this region with different amounts of fat and scanty pancreatic lobules, but also the fact that this area is dissected in a more blunted manner by the surgeons. Some surgeons use staples in this area. In that case, peri-staple sampling is the best a surgical pathologist can do. In addition to fat, this area also invariably contains LNs, which is not the case in the vascular bed or the pancreatic neck margin.
- CBD margin: The CBD travels antero-laterally to the uncinate margin, and the CBD margin can be localized at the plateau of the trapezoid's upper right edge. If it could not be identified due to suturing (Fig. 2), a cut of the plateau allows its edge to open up. In that case, this cut fragment would serve as the en face CBD margin. The lumen is typically wider than several millimeters. It may contain a stent. If this margin reveals a double duct (goggle appearance), it means the cystic duct has also been included in the specimen. Depending on the type of the operation, not only a segment of cystic duct but the whole gallbladder may be attached to the CBD (Fig. 4), in which case technically the cut margin would be the hepatic duct margin. An LN that is fairly large and often blood-rich (hemo-LN) is often present adjacent to the CBD. It so happens that despite often being large, this LN seldom proves positive for cancer, with the exception of some CBD carcinomas.
FIGURE 4.
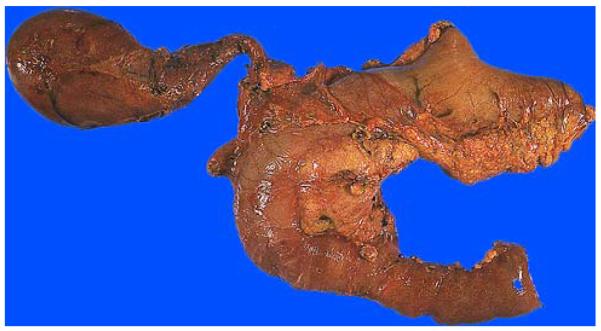
PD specimen from the anterior perspective. In this case, the gallbladder and part of the stomach were also removed en bloc. The pancreatic head often has abundant fat in the anterior surface and is localized to the curved part of the “C”-shaped duodenum.
Further Surrogate Clues to Orientation
Identification of the trapezoid and the features described above allow the identification of the pertinent structures in virtually all cases, and they also provide the simplest way for the orientation of the PD specimen. If other verifications are needed, the following clues can be considered:
Anterior pancreaticoduodenal junctional region: The anterior free surface typically contains abundant adipose tissue and is convex in appearance (Fig. 3A). In fact, there is often big collection and puckering of fat toward the superior aspect where omental tissue, if present, may also merge with this. Removal of this fat pad before further dissection may ease the identification of other structures. The transition from the pancreatic head to the duodenal wall is also often more irregular and abrupt compared with that in the posterior aspect (Fig. 3B), and, in fact, the edge of the pancreatic head often overarches on top of the duodenal wall, showing a palpable sharp edge. This surface is regarded as a margin by some authors (Table 1). We designate it as anterior free surface.
Posterior pancreaticoduodenal junctional region: In contrast to the anterior aspect, at the postero-lateral aspect of the specimen, there is a relatively flat and shiny region, in which the pancreatic head adjoins the duodenum (Fig. 3B). The transition is fairly smooth with the pancreaticoduodenal groove only barely identifiable in this alliance. This surface is regarded as a margin by some authors (Table 1). We designate it as posterior free surface.
Inferior region: This region is difficult to define because it is rather rounded and highly variable by individual, and the anterior surface merges imperceptibly with the posterior vascular bed.
Portal vein: In some cases, a portion of the portal vein is included in the specimen. This is typically localized to the mid-lower aspect of the vascular bed (the center of the trapezoid) and is attached to the pancreatic head with its anterior surface. It may or may not be present as a complete tube in the specimen.
Distal (intra-ampullary) segments of the ducts: The exact pattern of the intra-ampullary end of the main pancreatic duct and its alliance with the very distal segment of the CBD and how they connect to the duodenum is often difficult, if not impossible, to document unless a special method such as specimen endoscopic retrograde cholangiopancreatography (ERCP) or dye injection is performed. In other words, whether there is a “common channel” or a septum in between the 2 ducts and, if so, its length are parameters difficult to document in daily practice; however, we do not believe this is required for surgical pathology reports.
Cystic duct: Depending on the operation, the cystic duct (with or without the gallbladder) may be present, and its examination becomes important especially for cases in which the cystic duct adjoins the CBD within the pancreas proper, as the main pathology may be localized in this junction (low-union).22
Accessory duct and possible pancreas divisum: In some cases, a probe through the pancreatic duct may traverse to the minor (accessory) ampulla. This may either be the persistence of the accessory duct or a reflection of pancreas divisum, which can only be confirmed with functional studies or ERCP findings.
DISSECTION AND SAMPLING
There are different approaches to the dissection of the pancreatic head, with different advantages and disadvantages.9,10,13,23–25 The main focus of any approach applied ought to be to document the interactions of the pathologic conditions with the main anatomic structures and the pertinent margins.
We have found the following approach to be a good balance between complete but also practical and accurate evaluation of PD specimens that can be adopted and used with relative ease by ordinary gross room personnel in a conventional gross room and using routine procedures.
External Examination and Obtaining Margins
Examination of the Specimen Externally
It is necessary to be familiar with the clinical and radiologic findings before the dissection and to palpate and, if possible, identify the tumor externally, as this may occasionally change the approach. For example, if it is a proximal CBD cancer, inking of the radial soft tissue surfaces (radial margins) surrounding the CBD at the superior aspect (including the posterior free surfaces) is necessary as the proximal segment of the CBD does not have any surrounding pancreatic tissue in this area, Or if a cyst is palpated and if examination of fixed specimen is preferred over fresh examination, injecting formalin into the cyst and fixing the specimen to dissect at a later time can be considered. Tumor measurement may also have to be performed before the cyst is accidentally ruptured. In addition, sometimes, cystic lesions such as intraductal papillary mucinous neoplasm (IPMN) protrude from the uncinate margin, and, in such cases, dissection of this margin can be deferred after bisection of the head. If a smooth-contoured mass bulging from the pancreas is noted, this may indicate a lesion such as neuroendocrine tumor or solid-pseudopapillary neoplasm. Then, the location of the bulge (as to anterior or posterior free surfaces) can be recorded, inked separately, and submitted with perpendicular sections. Same is applicable for any tumor that is overtly involving the surfaces discussed previously. For tumors that appear to be ampullary, it is important to ink the serosa at the pancreaticoduodenal junction with a separate color and keep this area intact during the removal of LNs (discussed below) and later take perpendicular sections from the tumor, going to this area.
Optional Superficial Sampling of a Palpable Tumor for Banking
Tumor banking is increasingly becoming important. If it is intended to obtain fresh tumor for tumor banking, and if there is a palpable tumor, before inking or doing anything to the specimen, we orient it and make a small cut into this palpable tumor from an angle not to interfere any of the margin evaluation or other parameters discussed below and take a section for the bank. Then we carefully ink the edges of this region and document what has been done, to be relayed to the main prosector of the specimen.
If there is a cystic tumor and if cyst fluid collection is being planned for research purposes, it may be preferable to aspirate the fluid before the dissection of the specimen.
Removal of the Margins and Surfaces
Our approach involves sampling of the key margins before further sectioning of the specimen. In our opinion, this allows simple complete examination of the pertinent margins and avoids the hassle of trying to re-identify the margins after the specimen is cut. For this purpose, we first remove the following (Fig. 5):
CBD margin: The CBD margin is shaved and submitted en face.
Cystic duct edge: The cystic duct edge, which is present only in some cases (if the gallbladder is removed separately), is shaved and submitted en face.
Pancreatic neck margin: The pancreatic neck margin is shaved and submitted en face. This reflects the complete neck resection region. It is different (covers wider area) from the “duct” margin obtained specifically from the duct itself by some surgeons, which needs to be reconciled in a comment, accordingly, in the final diagnosis.
Uncinate margin: We sample this margin through the approach advocated by Luttges et al.7 We ink the uncinate margin, cut as a 3- to 5-mm-thick slice, and then bread-loaf and submit entirely as a perpendicular margin (Fig. 5), which typically takes 2 to 3 cassettes. We find this region to be a preferential area for the cancers to spread into. There are many explanations for this. This is a fatty region that allows insidious cancers to find homage (path of least-resistance phenomenon). Moreover, carcinoma involvement in other regions such as the vascular bed is more readily evident, appreciable by palpation or gross examination, or often renders the case “unresectable,” whereas carcinomas extending to the uncinate region are often highly insidious and not grossly visible,26,27 requiring it to be deferred to microscopic examination with thorough sampling.
Vascular bed (superior mesenteric/portal vein margin): In our experience, when carcinoma is close to this region, which is typically a compact less-fatty pancreatic tissue, it is readily evident by gross examination, unlike the situation in uncinate margin or free surfaces, which are a lot more adipose tissue rich, and renegade carcinoma clusters are often grossly inapparent. For vascular bed, we ink, take 1 perpendicular section from this area with the closest tumor, and report it as vascular bed surface, although some authors regard this as a margin (Table 1).
Gastric/duodenal margins: Obviously, the proximal duodenal/gastric margin is only very rarely involved, and the distal duodenal transection margin is virtually always irrelevant unless there is a separate pathology. Therefore, it is adequate to have only 1 representative section from each, which can also serve as mere documentative sampling of these sites as well as normal tissue for future molecular tests.
Anterior and posterior free surfaces: We sample these as shaved surfaces as a part of the orange-peeling approach for LN harvesting, which is discussed in detail below.
FIGURE 5.
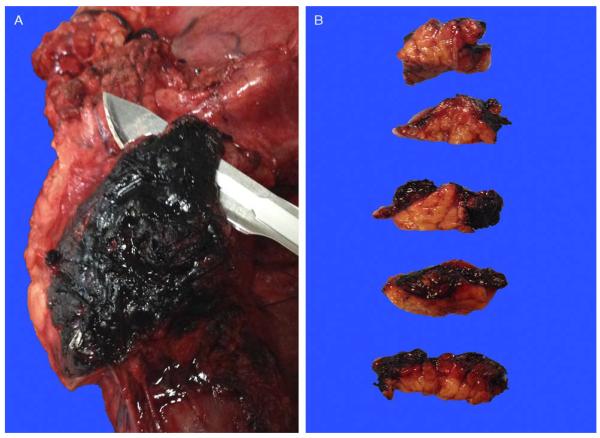
Our approach to the uncinate margin is to sample it thoroughly as a perpendicular margin, because there is often subtle and nonpalpable invasion into this adipose-rich area. Accordingly, this margin is inked black, cut into a 3 to 5-mm-thick slice (A), bread-loafed, and entirely submitted on edge as a perpendicular margin (B).
For the sake of completeness, we evaluate these margins regardless of whether they have been already sampled separately by the surgeons intraoperatively. In such cases, however, if the tumor is extending to the margin in these subsequent sections, we report it as an “edge” and refer to the appropriate parts of the specimen for the “true margin” by noting: “Tumor extends to the–edge of the specimen (please see the pertinent part for the true margin).” It is noteworthy that the value of a frozen section is dubious for any of these margins, with the exception of diseases like intraductal neoplasms.
As discussed above, whether to regard the posterior and anterior surfaces as anatomic “surfaces” or true margins is hotly debated. We favor the former approach. However, regardless of what name (surface, margin, or other), it is important to document their involvement, because it is presumed to be significant for the risk assessment of local tumor spread/recurrence. For example, we would designate a case as “Surgical margins are negative for carcinoma; however, the carcinoma extends to the anterior free surface,” so on and so forth.
Other Pertinent Structures Present Externally: Portal Vein
If present, portal vein ought to be sampled to document (1) the involvement of the vein by carcinoma, by taking perpendicular section(s) to the vein wall. Inking of both the adventitial and the endovascular surfaces in different colors may help the orientation at the microscopic level, which often proves to be harder than one thinks. (2) The superior and inferior edges of the vein have to be sampled as they also technically constitute resection margins, by either taking shaved margins of each edge or including them into the main section. If the latter is preferred, again inking of both the superior and inferior edges in different colors helps the orientation.
Opening and Sectioning
Opening the Duodenum; Examining Intraduodenal Pathology
After obtaining the margins, we open the duodenum to examine any intraduodenal pathology. We usually open the duodenum through the antiampullary (antipancreatic) edge (Fig. 6), unless there is a palpable tumor at this edge that might change our approach. Once the duodenum is opened, the ampulla should be readily visible (Fig. 7). Accessory ampulla is also identifiable as a small bump, usually located about 2 cm proximal and anterior to the ampulla and appears as a tiny bump, which can be accentuated by folding and stretching the specimen from the edges to allow this structure stand out further.
FIGURE 6.
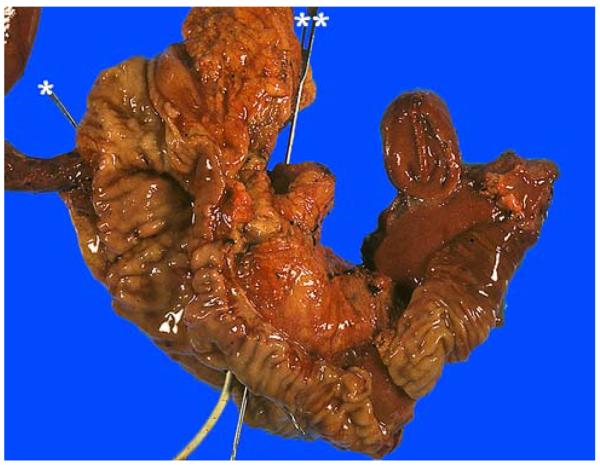
After obtaining the margins, we open the duodenum through the antiampullary (antipancreatic) edge to examine the ampulla. The pancreatic (*) and common bile (**) ducts are probed. There is also a stent (white) in the CBD in this case.
FIGURE 7.
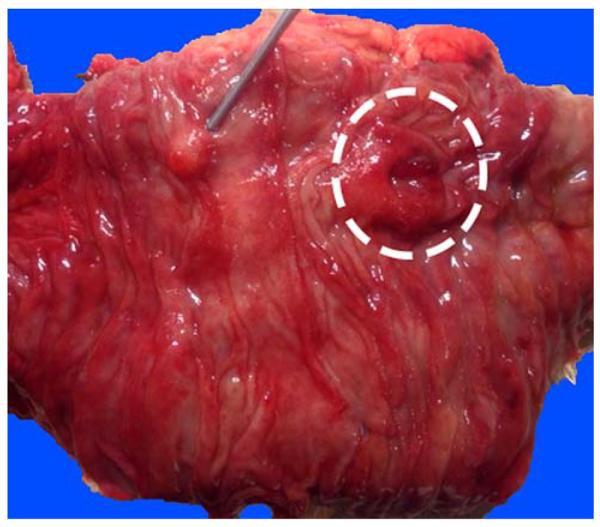
Although its anatomy varies between individuals, from the duodenal aspect, the major ampulla (dashed circle) is usually readily visible. The accessory ampulla (probe), typically located about 2 cm proximal and slightly anterior to the papilla of Vater, appears as a nodule and can be mistaken for a polyp endoscopically. It is important to examine this area for any mucosal nodularities or irregularities or thickening, as paraduodenal pancreatitis often manifests in this region with variable, often subtle, abnormalities.
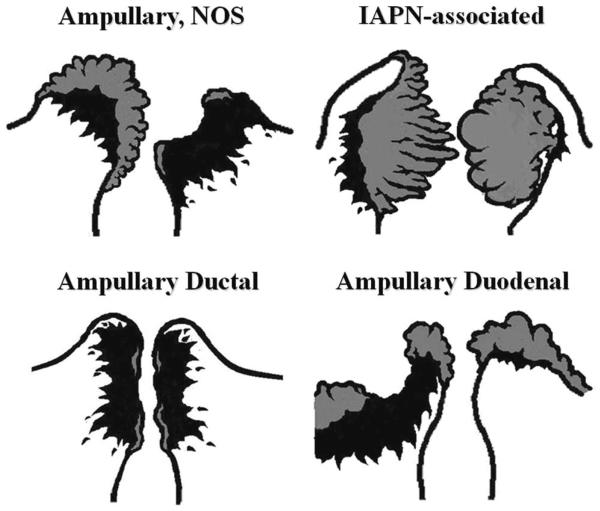
Examination of the ampulla from the duodenal aspect is crucial for proper classification of an ampullary tumor into the 4 recently recognized categories28 of ampullary carcinomas (Fig. 8). This often requires the probing of the CBD to orient the ampullary orifice, especially if this area is effaced by a tumor (see below for details).
FIGURE 8.
For tumors arising in the ampullary region, careful gross examination of the distribution of the tumor and determination of the preinvasive component (adenomatous lesions that typically manifest as granular, feathery, friable, or smooth-surfaced nodular material; highlighted by gray color in this diagram) are crucial for the proper classification of the ampullary tumors. Ampullary region carcinomas comprise 4 distinct types: Intra-ampullary papillary tubular neoplasm (IAPN)-associated carcinomas are characterized by a significant preinvasive component that grows as an exophytic mass within the ampullary channel (the distal tip of the CBD and the main pancreatic duct). Carcinomas of ampullary ducts are predominantly invasive tumors that circumferentially constrict the distal end of the CBD and pancreatic duct, with minimal changes of the papilla of Vater and ampullary duodenum mucosa. Ampullary duodenal carcinomas usually arise from an adenoma of ampullary duodenum, forming bulky lesions in which the ampullary orifice is often eccentrically located. Tumors that arise from the papilla of Vater itself, as well as those not showing features that characterize the other 3 groups, are classified as ampullary carcinoma, not otherwise specified (NOS).
Special attention to the accessory ampullary region is also warranted. If mucosal nodularities or puckering are identified here without any significant ulceration, the possibility of paraduodenal (groove) pancreatitis29 ought to be considered very seriously and the dissection to be focused in this region and adjacent pancreas. In fact, macroscopic examination is crucial for the diagnosis of this entity.
Dissection of the LNs (by the Orange-peeling Method)
LNs of a PD specimen are embedded into the surfaces and creases and are much easier to dissect before the sectioning of the pancreatic head. Searching for LNs after the head is dissected becomes much more difficult and leads to lower yield. Although any method to allow complete identification of LNs is perfectly valid, the approach we have taken to ensure thorough removal of the LNs is the orange-peeling approach, in which we shave off all the free surfaces of the pancreatic head that potentially harbor LNs (Fig. 9) and separate them into 7 arbitrary regions, which more or less correspond to the LN groups as well: Peri-CBD, anterior pancreatic, anterior pancreaticoduodenal, superior pancreatic, inferior pancreatic, posterior pancreatic, posterior pancreaticoduodenal. More than anything else, these arbitrary regions serve as a checklist to ensure complete identification. After inking (preferably in 2 different colors such as blue for anterior free surface, black for posterior free surface), these free surfaces are peeled off with the help of either a scissors or a blade, and, even if no LN is identified, all fragments are submitted entirely in an attempt to identify microscopic LNs. These sections also serve as samplings of the external soft tissues covering the pancreas, which presumably correspond to what must have been intended as “peripancreatic soft tissue” in the current American Joint Committee on Cancer TNM, although there really is no such definable structure in this organ, because of irregularity of the surfaces, the lack of a capsule, and abundance of fat between pancreatic lobules. In fact, in 90% of the pancreatic ductal adenocarcinoma cases, foci of carcinoma (most are not evident grossly) are identified in these sections (pT3) microscopically.26,27,30
FIGURE 9.
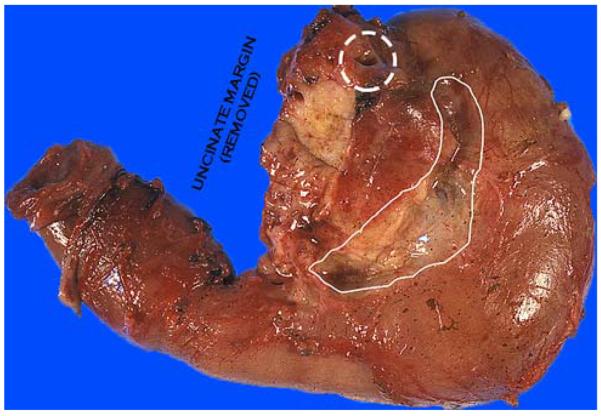
The orange-peeling method. The soft tissues covering the pancreas are shaved off after the margins have been obtained and the ampulla examined, before the sectioning of the pancreatic head. This figure illustrates the view after the posterior pancreaticoduodenal LN area (groove between pancreatic head and duodenal wall) has been shaved off. These fragments are searched for LNs and, even if no LN is identified, submitted entirely. These fragments also serve as the documentation of peripancreatic soft tissues, which often yields subtle cancerous foci (isolated solitary ducts). Note the CBD orifice at the superior edge, at the plateau (dashed circle).
These orange-peeled soft tissues also serve as shaved samples of some of the areas that are considered as “margin” by other authors (Table 1): The anterior and posterior free surfaces, which are sampled in the anterior pancreaticoduodenal-anterior pancreatic sections and posterior pancreaticoduodenal (Fig. 9)-posterior pancreatic sections, respectively. If carcinoma cells are identified in these sections extending to the ink, it is reported as surface (or “margin”) involvement. If the carcinoma cells are not touching the ink, then it is reported as close.
Counting the number of LNs may have to be modified according to the method they are sampled with. As the orange-peeling method is essentially an LN dissection approach, each LN identified typically reflects 1 LN, with the exception of the retroperitoneal region, which we sample as perpendicular serial sections, and thus the LNs that appear in each microscopic section may reflect a different portion of the same LN. We attempt to discount this accordingly. Consecutive sectioning of the margin often helps identify and thus discount continuous LNs. This type of corrective attempt would have to be used more extensively if the LNs are a part of vertical sampling, such as in the Verbeke, Esposito and Maksymov protocols, in which the (posterior) free surface(s) are sampled perpendicularly.9–11,13,19–21,31
Sectioning of the Pancreas, Ducts, and Ampulla
There are various approaches in sectioning the pancreatic head, and each has its own advantages.9,10,13,21–23,29,30 We find the bivalving of the pancreatic head through the plane that goes through both ducts to be the most informative, allowing us to examine and document essentially any pathologic condition thoroughly and accurately. This approach involves probing of both the pancreatic duct and CBD (Fig. 6). More importantly, this approach allows proper classification and staging of ampullary cancers (Fig. 8; see below).
Probing of CBD and pancreatic duct
The CBD, unlike the pancreatic duct, is virtually always probe-patent even in the presence of constrictive tumors, presumably because of its large size and elasticity, as well as the lack of any collaterals, thus its total obstruction being incompatible with life/operability. The pancreatic duct, in contrast, may be difficult to probe and trace throughout its length. Although it is typically probable in the very proximal third, the distal part proves to be problematic in about a third of the cases. This is partly because of the presence of a kink, referred to as the “Wirsungian knee,” and partly because of the fact that the pancreatic duct is often really narrow (at the range of 1 to 2 mm in diameter) in this region, and the probes that are in common use are typically far too thick for this purpose. However, even when we used very thin probes, this area was difficult to disclose in routine practice. Another major factor is the presence of a compressing tumor, which can occlude the pancreatic duct to virtual invisibility. If the pancreatic duct is not probe-patent, one possibility would be to try to find the orifice of the pancreatic duct from the ampullary region and to retro-probe it from there. This may require cutting of the CBD to identify the pancreatic duct orifice at the edge.
In some cases, the probe inserted into the pancreatic duct comes out in the duodenum 1.5 to 2 cm proximally. That reflects the accessory duct of Santorini and raises the possibility of pancreas divisum, which may have to be confirmed/verified by the clinical findings, in particular, ERCP. In such cases, careful inspection of the trace of this duct may yield to the identification of a connection with the main (Wirsung) duct.
Bivalving the pancreatic head along the probes
Once the probes are placed in the ducts (all the way to the ampulla for the CBD and as far as it can be traced for the pancreatic duct), the pancreatic head is then bivalved along the plane that goes through both of these ducts and the ampulla (Fig. 10). To accomplish this, the knife needs to be angled as if cutting the probes themselves. Then, the ducts are cut using broad strokes (violin bow strokes), and the tissue is examined after each stroke, to assess and re-angle accordingly to ensure the tracing of both ducts in the plane of cut. As approaching to the ampullary region, it may be necessary to make cuts to the duodenum to release the plane of the ampulla, because the duodenum typically folds and resists to cutting at the intended plane.
FIGURE 10.
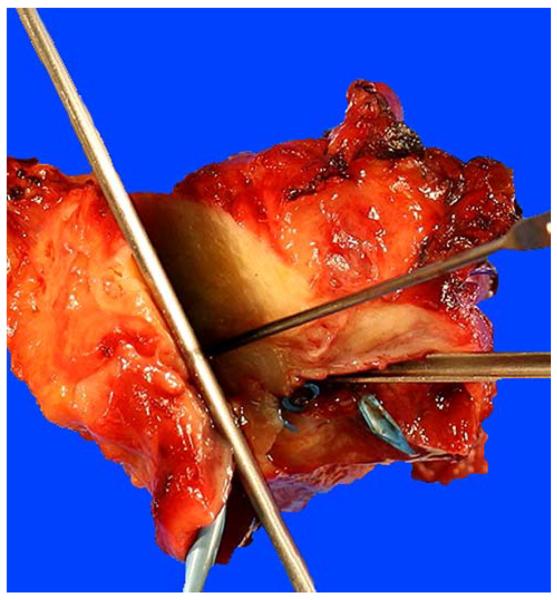
This image illustrates the bivalving of the pancreatic head after both ducts are probed. There is also a stent (blue) in the CBD. With every cut made, the prosector re-checks whether both ducts are still in the same plane. In this case, the knife would have to be re-angled to re-include the CBD.
Evaluation of the bivalved specimen and further dissection
From the luminal aspect, CBD is often light yellow (bile-stained) and displays numerous tiny punctuate orifices of the sacculi of Beale (peribiliary glands). It appears rougher than the pancreatic duct, which shows a more pink-white, smooth-glistening appearance (Fig. 11). In virtually all cases, CBD is significantly larger than the main pancreatic duct.
FIGURE 11.
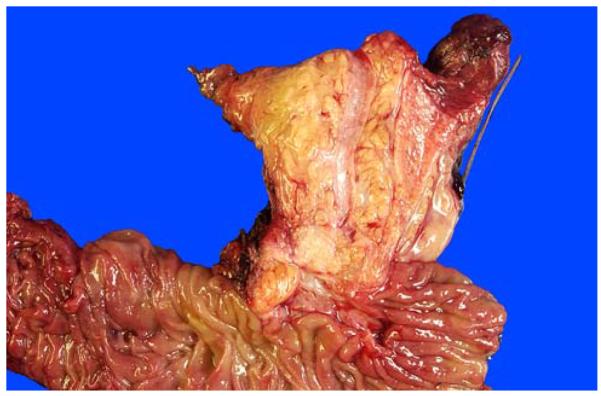
Bivalved pancreatic head before fixation. The CBD is significantly larger and light yellow, in the mucosa of which punctate orifices of peribiliary glands are visible on close inspection. The pancreatic duct is usually pink-white with a smooth-glistening appearance and is typically much narrower. Sectioning with this approach allows the evaluation of the compartments of the ampulla (intra-ampullary mucosa, distal segments of the CBD and pancreatic duct, and the duodenal mucosa/wall) as one continuous tube. Thus, distribution of the lesions, not only among these structures but also into their thin walls, can be readily appreciated.
Bivalving of the pancreatic head at the plane of both ducts allows the assessment of the distribution of the tumors in relation to these ducts (Fig. 11). It has other major advantages in assessing especially the primary ampullary tumors.28 First of all, with this approach, it is possible to evaluate the compartments of the ampulla (intra-ampullary mucosa, the distal segment of the CBD and pancreatic duct, and the duodenal mucosa/wall), all as if they are segments of one continuous tube-like structure (Fig. 11). For tumors specifically arising from the papilla of Vater (the edge of the ampulla where the duodenal mucosa joins the distal ends of the ducts), these sections allow better documentation of the mucosal spread of these tumors to the neighboring CBD and duodenum, whereas, with amputative (axial/transverse) sampling of the ampulla, this relationship cannot be documented. More importantly, this approach allows the proper documentation of an ampullary tumor invading radially into the groove area where the ampulla is not covered by the pancreas (Fig. 12), to which ampullary tumors often get ready access, invading to the periduodenal soft tissues and duodenal serosa, without having to go through the pancreas.27,28,32
FIGURE 12.
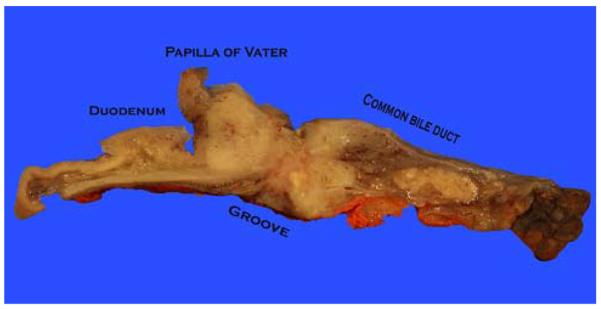
The ampulla is not covered by the pancreas at the pancreaticoduodenal junction (groove), where ampullary tumors often invade into the periduodenal soft tissues and duodenal serosa without having to go through the pancreas. Therefore, for proper staging of ampullary tumors in this plane, it is important to ink the serosa at the groove area and take perpendicular sections (from tumor to the serosa) to document the tumor spread to this region, which is common in our experience.
In addition, with this approach any pathology in the ducts (tumor stricture, papillary units, cystic dilatations, stone formation, etc.) can be documented adequately.
Macroscopic Clues to the Tumor Recognition
Most PDs are performed for pancreatobiliary-type adenocarcinomas. These are scirrhous, ill-defined lesions. They typically show a gray-green hue. Some are more firm-white. Punctate necrosis can be seen. Cystic degeneration is uncommon. Milky-homogenous appearance is more characteristic of pseudotumors (see below). When they infiltrate the surrounding soft tissue, they form illdefined lesions and puckering of the adipose tissue.
Intraductal neoplasms often present as cystic tumors that may be filled with granular hemorrhagic material.33 Their intraductal nature is often presumed rather than documentable anatomically. However, for branch duct–type IPMNs, which form multilocular cystic tumors, it is important to document the findings in the main pancreatic duct. This can only be accomplished by proper gross examination, inking, and sampling of the main pancreatic duct, because, microscopically, there is nothing specific to the main pancreatic duct to distinguish it from dilated or larger branch ducts. Furthermore, if there is a dilatation in the main duct, it is important to document not only the mucosa of the duct but also the duct wall, especially if there is an area with constriction, because small lesions, such as neuroendocrine tumors may, present with ductal obstruction and a pseudo-IPMN pattern.34–36
Proper gross examination and dissection can also help reduce the cost by more targeted sampling. For example, sponge-like configuration of microcystic serous cyst adenomas is so characteristic that very limited sampling of these tumors is adequate. In contrast, cystic lesions with mucinous epithelium are precancerous and need to be examined thoroughly, if not entirely. However, if complex and solid areas are identified and sampled in the first run, then an invasive carcinoma can be documented readily without having to sample the entire lesion from the get go. In addition, granular and nodular areas of these tumors often correspond to papillary nodules, potentially with high-grade transformation.
Nonductal tumors (neuroendocrine tumor, solid-pseudopapillary neoplasm, acinar cell carcinoma, pancreatoblastoma, and mesenchymal and secondary tumors) often form a more demarcated lesion, and they typically have a more fleshy appearance because of their cellularity and lack of desmoplastic stroma.
As mentioned previously, proper examination and dissection is crucial for the diagnosis of ampullary carcinomas and their classification into 1 of the 4 recently recognized categories28 (Fig. 8). Whether the bulk (> 75%) of the tumor is located in the ampulla is the main definition of ampullary carcinoma. If there is a florid vegetating/ulcerating mass on the duodenal surface of the ampulla and the ampullary orifice is eccentrically located in the lesion this is most likely a carcinoma of the ampullary duodenum. Intra-ampullary papillary tubular neoplasms37 are characterized by a centrally located orifice and granular/nodular material filling the ampullary channel and exuding into the duodenal surface. Carcinomas of ampullary ducts are usually subtle from the duodenal perspective and, on cut sections, reveal plaque-like circumferential scarring of the distal end of the ducts by an insidious infiltration. It should also be noted here that, in about a third of ampullary cancers, the bulk of the tumor is composed of preinvasive (adenomatous) lesions. Gross examination is crucial in determining the presence of invasive cancer and its size and extent. The size of the invasive carcinoma ought to be documented separately from the size of the preinvasive component in these lesions.
Classification of a carcinoma according to the CBD origin is also almost entirely reliant on the growth pattern of the tumor as observed grossly. We classify a lesion as primary CBD origin (rather than a pancreatic ductal carcinoma) only if the bulk of the lesion is circumferentially around the CBD as documented grossly and proved microscopically with proper sampling. In our experience,38 only 5% of the PDs in the West contain such tumors; however, this is much more common in Asia.
Identification of nonampullary duodenal carcinomas, which are proving to be a very unique group with unexpected characteristics,39 also requires careful grossing documentation. If they occur in the nonpancreatic segment of the duodenum, they are relatively easy to recognize. However, those occurring close to the ampulla or ulcerating into the pancreas need careful documentation of the epicenter of the lesion, its wedge-shape with the base located on the duodenal mucosa, and also, by definition, the preservation (uninvolvement) of the ampulla. It is noteworthy that nonampullary duodenal carcinomas that have a plaque-like configuration appear to have a close association with microsatellite instability, another important contribution of proper grossing.
About 5% of the PDs performed with the clinical diagnosis of “cancer” prove to be pseudotumors.29,40 Many of these are paraduodenal (groove) pancreatitis,29 which requires careful examination of the accessory ampulla region as discussed above. Other forms of pseudo-tumors include adenomyomatous hyperplasia of the ampulla or sclerosing choledochitis, which also require careful examination and sampling of these structures because these are often very subtle processes.40 For lipomatous pseudohypertrophy, documentation of the demarcation of the adipose tissue from the surrounding tissue is an important clue to the diagnosis.41
Microscopic Verification; Considerations
It should be kept in mind that it can be difficult to distinguish the CBD and main pancreatic duct microscopically. The CBD is generally thicker and wider and contains a lot more peribiliary mucous glands (sacculi of Beale, which appear as punctate orifices) than the tributary ductules on the main pancreatic duct wall, but depending on the pathology and injury these can vary. In addition, the CBDs have pancreatic tissue only in the more distal segments and only semilunarly. However, with the bivalving approach described above, this can be difficult to verify at times because the duct has been opened and sectioned from one side only. For this reason, after the bivalving of the head, we typically apply different colors to the CBD and main pancreatic duct so that in the sections they can be distinguished, if necessary, which becomes handy in some cases. Moreover, it should be reiterated here that there is no distinguishing feature of the main pancreatic duct from branch ducts, and, therefore, documentation relies on the gross findings and proper sampling. Inking of the main pancreatic duct mucosa also helps in this regard.
SUMMARY
PD specimens are fairly complex. Their proper orientation, dissection, and sampling are crucial for the accurate diagnosis, classification, and staging of tumors and identification of pseudotumors that are removed with this operation. There are various approaches in grossing these specimens, and each has its advantages and disadvantages. It is important to be aware of the pitfalls so that precautions can be taken to averse them. Presented above is our experience based on 1080 PD dissections performed by us. We believe our approach accomplishes all the requirements of tumor diagnosis and documentation without going to excessive cost.
ACKNOWLEDGMENTS
The authors thank Allyne Manzo and Lorraine Biedrzycki for assistance with the figures.
Source of Funding: Supported in part by the National Cancer Institute Specialized Program in Research Excellence (SPORE) CA62924 and in part by the Georgia Cancer Coalition Distinguished Cancer Clinicians and Scientists Program. N.V.A. has a grant from National Institutes of Health (#5P50 CA62924).
Footnotes
Conflicts of Interest For the remaining authors none were declared.
REFERENCES
- 1.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 2.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruzoni M, Johnston E, Sasson AR. Pancreatic incidentalomas: clinical and pathologic spectrum. Am J Surg. 2008;195:329–332. doi: 10.1016/j.amjsurg.2007.12.027. discussion 332. [DOI] [PubMed] [Google Scholar]
- 4.Hopt U, Keck T. Pancreatic incidentalomas. Correct assessment and therapy. Chirurg. 2007;78:713–720. doi: 10.1007/s00104-007-1373-x. [DOI] [PubMed] [Google Scholar]
- 5.Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133:423–438. doi: 10.5858/133.3.423. [DOI] [PubMed] [Google Scholar]
- 6.Staley CA, Cleary KR, Abbruzzese JL, et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12:373–380. doi: 10.1097/00006676-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Luttges J, Zamboni G, Kloppel G. Recommendation for the examination of pancreaticoduodenectomy specimens removed from patients with carcinoma of the exocrine pancreas. A proposal for a standardized pathological staging of pancreaticoduodenectomy specimens including a checklist. Dig Surg. 1999;16:291–296. doi: 10.1159/000018738. [DOI] [PubMed] [Google Scholar]
- 8.Chatelain D, Flejou JF. Pancreatectomy for adenocarcinoma: prognostic factors, recommendations for pathological reports. Ann Pathol. 2002;22:422–431. [PubMed] [Google Scholar]
- 9.Verbeke CS, Gladhaug IP. Resection margin involvement and tumour origin in pancreatic head cancer. Br J Surg. 2012;99:1036–1049. doi: 10.1002/bjs.8734. [DOI] [PubMed] [Google Scholar]
- 10.Verbeke CS, Leitch D, Menon KV, et al. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 11.Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB (Oxford) 2009;11:282–289. doi: 10.1111/j.1477-2574.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Royal College of Pathologists of United Kingdom Standards and Datasets for Reporting Cancers. [Accessed December 24, 2013];Data Set for the Histopathological Reporting of Carcinomas of the Pancreas, Ampulla of Vater and Common Bile Duct. 2013 Available at: http://www.rcpath.org/NR/rdonlyres/954273A2-3F01-4B97-B0F6-C136231DF65F/0/dataset histopathologicalreportingcarcinomasmay10.pdf.
- 13.Maksymov V, Hogan M, Khalifa MA. An anatomical-based mapping analysis of the pancreaticoduodenectomy retroperitoneal margin highlights the urgent need for standardized assessment. HPB (Oxford) 2013;15:218–223. doi: 10.1111/j.1477-2574.2012.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakao A, Takeda S, Sakai M, et al. Extended radical resection versus standard resection for pancreatic cancer: the rationale for extended radical resection. Pancreas. 2004;28:289–292. doi: 10.1097/00006676-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Yeo CJ, Cameron JL, Sohn TA, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg. 1999;229:613–622. doi: 10.1097/00000658-199905000-00003. discussion 622–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riall TS, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma—part 3: update on 5-year survival. J Gastrointest Surg. 2005;9:1191–1204. doi: 10.1016/j.gassur.2005.08.034. discussion 1204–6. [DOI] [PubMed] [Google Scholar]
- 17.American Joint Committee on Cancer . Exocrine pancreas. In: Edge S, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. Springer; Chicago: 2009. pp. 241–249. [Google Scholar]
- 18.College of American Pathologists [Accessed December 24, 2013];Pancreas (exocrine) 2012 Available at: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2013/PancreasExo_13protocol_3201.pdf.
- 19.Verbeke CS, Gladhaug IP. Authors' reply: Resection margin involvement and tumour origin in pancreatic head cancer (Br J Surg 2012; 99: 1036–1049) Br J Surg. 2013;100:299. doi: 10.1002/bjs.8734. [DOI] [PubMed] [Google Scholar]
- 20.Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- 21.Schlitter AM, Esposito I. Definition of microscopic tumor clearance (R0) in pancreatic cancer resections. Cancers (Basel) 2010;2:2001–2010. doi: 10.3390/cancers2042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez RS, Saka B, Bagci P, et al. Neoplasms and pseudotumors associated with low (intrapancreatic) union of cystic duct into common bile duct: a clinicopathologic analysis of 15 cases of a hitherto unrecognized phenomenon (abstract) Mod Pathol. 2013;26(2S):424A. [Google Scholar]
- 23.Adsay NV, Basturk O, Altinel D, et al. The number of lymph nodes identified in a simple pancreatoduodenectomy specimen: comparison of conventional vs orange-peeling approach in pathologic assessment. Mod Pathol. 2009;22:107–112. doi: 10.1038/modpathol.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalifa MA, Maksymov V, Rowsell C. Retroperitoneal margin of the pancreaticoduodenectomy specimen: anatomic mapping for the surgical pathologist. Virchows Arch. 2009;454:125–131. doi: 10.1007/s00428-008-0711-9. [DOI] [PubMed] [Google Scholar]
- 25.Porembka MR, Hawkins WG, Linehan DC, et al. Radiologic and intraoperative detection of need for mesenteric vein resection in patients with adenocarcinoma of the head of the pancreas. HPB (Oxford) 2011;13:633–642. doi: 10.1111/j.1477-2574.2011.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandyopadhyay S, Basturk O, Coban I, et al. Isolated solitary ducts (naked ducts) in adipose tissue: a specific but underappreciated finding of pancreatic adenocarcinoma and one of the potential reasons of understaging and high recurrence rate. Am J Surg Pathol. 2009;33:425–429. doi: 10.1097/PAS.0b013e3181908e42. [DOI] [PubMed] [Google Scholar]
- 27.Adsay NV, Bagci P, Tajiri T, et al. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. 2012;29:127–141. doi: 10.1053/j.semdp.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Adsay V, Ohike N, Tajiri T, et al. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. 2012;36:1592–1608. doi: 10.1097/PAS.0b013e31826399d8. [DOI] [PubMed] [Google Scholar]
- 29.Adsay NV, Zamboni G. Paraduodenal pancreatitis: a clinicopathologically distinct entity unifying “cystic dystrophy of hetero-topic pancreas”, “para-duodenal wall cyst”, and “groove pancreatitis”. Semin Diagn Pathol. 2004;21:247–254. doi: 10.1053/j.semdp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Oliva I, Bandyopadhyay S, Coban I, et al. Peripancreatic soft tissue involvement by pancreatic ductal adenocarcinomas: incidence, patterns and significance (abstract) Mod Pathol. 2009;22:318A. [Google Scholar]
- 31.Rau BM, Moritz K, Schuschan S, et al. R1 resection in pancreatic cancer has significant impact on long-term outcome in standardized pathology modified for routine use. Surgery. 2012;152(suppl 1):S103–S111. doi: 10.1016/j.surg.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Adsay NV, Basturk O, Saka B. Pathologic staging of tumors: pitfalls and opportunities for improvements. Semin Diagn Pathol. 2012;29:103–108. doi: 10.1053/j.semdp.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumors of Digestive System. WHO Press; Lyon: 2010. [Google Scholar]
- 34.Kawamoto S, Shi C, Hruban RH, et al. Small serotonin-producing neuroendocrine tumor of the pancreas associated with pancreatic duct obstruction. Am J Roentgenol. 2011;197:W482–W488. doi: 10.2214/AJR.10.5428. [DOI] [PubMed] [Google Scholar]
- 35.Kenney B, Singh G, Salem RR, et al. Pseudointraductal papillary mucinous neoplasia caused by microscopic periductal endocrine tumors of the pancreas: a report of 3 cases. Hum Pathol. 2011;42:1034–1041. doi: 10.1016/j.humpath.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Shi C, Siegelman SS, Kawamoto S, et al. Pancreatic duct stenosis secondary to small endocrine neoplasms: a manifestation of serotonin production? Radiology. 2010;257:107–114. doi: 10.1148/radiol.10100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohike N, Kim GE, Tajiri T, et al. Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases. Am J Surg Pathol. 2010;34:1731–1748. doi: 10.1097/PAS.0b013e3181f8ff05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez RS, Bagci P, Kong KT, et al. Distal common bile duct adenocarcinoma: analysis of 47 cases and comparison with pancreatic and ampullary ductal carcinomas (abstract) Mod Pathol. 2012;25:109A. doi: 10.1038/modpathol.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saka B, Bagci P, Krasinskas A, et al. Duodenal carcinomas of non-ampullary origin are significantly more aggressive than ampullary carcinomas (abstract) Mod Pathol. 2013;26(2S):176A. [Google Scholar]
- 40.Adsay NV, Basturk O, Klimstra DS, et al. Pancreatic pseudotumors: non-neoplastic solid lesions of the pancreas that clinically mimic pancreas cancer. Semin Diagn Pathol. 2004;21:260–267. doi: 10.1053/j.semdp.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Altinel D, Basturk O, Sarmiento JM, et al. Lipomatous pseudohypertrophy of the pancreas: a clinicopathologically distinct entity. Pancreas. 2010;39:392–397. doi: 10.1097/MPA.0b013e3181bd2923. [DOI] [PMC free article] [PubMed] [Google Scholar]