Table 1. Scope of the catalytic diamination of 2-allylanilinesa.
| Entry | Substrate | RNH2 | Product | % Yield |
|---|---|---|---|---|
| 1 |
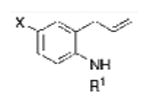 1a, X = H, R1 = Ms, |
TsNH2 |
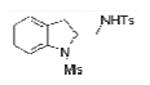 2a |
84 (75)b |
| 2 | 1a | MsNH2 |
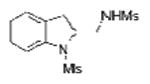 2b |
69 |
| 3 | 1a | SESNH2 |
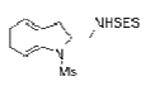 2c |
80 |
| 4 | 1a | BzNH2 |
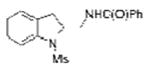 2d |
50 |
| 5 | 1b, X = H, R1 = Ts | TsNH2 |
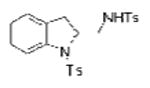 2e |
64c |
| 6 | 1c, X = H, R1 = SO2Bn | TsNH2 |
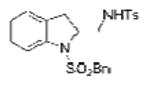 2f |
65 |
| 7 | 1d, X = H, R1 = SO2C3H5 | TsNH2 |
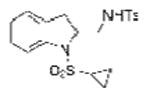 2g |
75 |
| 8 | 1e, X = H, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
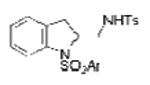 2b, Ar = 3,5-di-t-Bu-4-MeOC6H2 |
84 |
| 9 | 1e | SESNH2 |
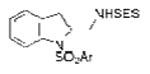 2i |
77 |
| 10 | 1f, X = OMe, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
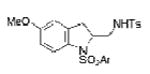 2j |
75 |
| 11 | 1g, X = Br, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
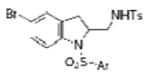 2k |
68 |
| 12 | 1h, X = Cl, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
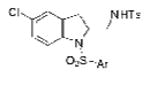 2l |
70 |
| 13 | 1i, X = F, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
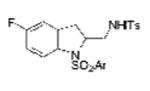 2m |
70 |
| 14 | 1j, X = Me, R1 = 3,5-di-t-Bu- 4-MeOC6H2SO2 | TsNH2 |
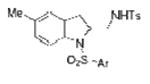 2n |
80 |
| 15d |
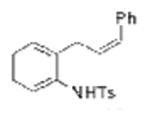 1k |
TsNH2 |
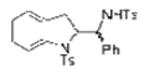 2o, 2p (dr = 58:42) |
76 |
| 16d |
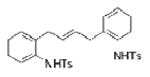 1l |
-- |
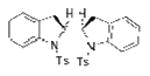 2q |
73 |
Conditions:
20 mol % Cu(2-ethylhexanoate)2, MnO2 (3 equiv), 2,6-di-t-Bu-4-Me-pyridine (1 equiv), PhCF3, RNH2 (2.2 equiv), 110 °C, 24 h.
Using 2 equiv (t-BuO)2 at 100 °C instead of MnO2.
31% of sultam 3 also isolated.
In entry 15, [Cu(bisoxazoline)](OTf)2 was used. For entry 16 [Cu(R,R)-Ph-box](OTf)2 was used. 8% KMnO4 and 1:1 PhCF3:1,2-dichloroethane solvent were used in both reactions. Entries 1-3 (except 1b) were taken from ref. 21.
