Table 5. Chiral 2-aminopyrrolidine formation via enantioselective alkene diamination.
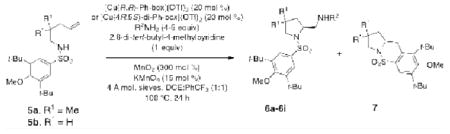
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Entry | Substrate | Amine Nucleophile | Product | Ratio 6:7 | Yield (%) 6 | ee (%) 6 |
| 1a | 5a | TsNH2 | 6a, R1 = Me, R2 = Ts | 67:33 | 64 | >95 |
| 2b | 5a | TsNH2 | 6a, R1 = Me, R2 = Ts | 76:24 | 72 | >95 |
| 3c | 5a | SESNH2 | 6b, R1 = Me, R2 = SES | 63:37 | 58 | >95 |
| 4c | 5a | BsNH2 | 6c, R1 = Me, R2 = Bs | 69:31 | 63 | >95 |
| 5c | 5a | 2-MeC6H4SO2NH2 | 6d, R1 = Me, R2 = SO2(2-MeC6H4) | 68:32 | 62 | >95 |
| 6c | 5a | 4-MeOC6H4SO2NH2 | 6e, R1 = Me, R2 = SO2(4-MeOC6H4) | 66:34 | 60 | >95 |
| 7c | 5a | 4-ClC6H4SO2NH2 | 6f, R1 = Me, R2 = SO2(4-ClC6H4) | 64:36 | 60 | >95 |
| 8c | 5a | 4-NO2C6H4SO2NH2 | 6g, R1 = Me, R2 = SO2(4-NO2C6H4) | 64:36 | 58 | >95 |
| 9c | 5a | C3H5SO2NH2 | 6h, R1 = Me, R2 = SO2C3H5 | 67:33 | 61 | >95 |
| 10c | 5b | TsNH2 | 6i, R1 = H, R2 = Ts | 66:34 | 61 | 89 |
Conditions:
Using the (R,R)-Ph-box ligand (22 mol %) with 20 mol % Cu(OTf)2 and 4 equiv R2NH2.
Conditions (a) except using the (R,R)-Ph-box ligand (33 mol %) with 30 mol % Cu(OTf)2.
Conditions (a) except using the (4R,5S)-di-Ph-box ligand (22 mol %) with 20 mol % Cu(OTf)2.
Conditions (c) except using 5 equiv R2NH2. SES = 2-trimethylsilylethylsulfonyl, Bs = benzenesulfonyl.
