Abstract
Membrane transporter PhT2 (SLC15A3), which belongs to the proton-coupled oligopeptide transporter family, mediates the transport of di/tripeptides and histidine utilizing an inwardly directed proton gradient and negative membrane potential. The aim of this study was to elucidate the molecular expression of PhT2 in macrophages and mouse tissues and to explore the regulation of PhT2 by lipopolysaccharide (LPS). The results showed relatively high expression of PhT2 in J774A.1 and THP-1 macrophage cells, mouse spleen, and lung. Using an LPS-induced inflammatory cell model, we found that hPhT2 mRNA expression was up-regulated in THP-1 cells and that the up-regulation was suppressed by pyrrolidine dithiocarbamate, a specific inhibitor of NF-κB. Similar results were observed in mouse spleen during LPS-induced acute inflammation. Using dual-labeling immunofluorescence and confocal laser scanning microscopy, we confirmed that mPhT2 was colocalizing with lysosome-associated membrane protein 1 in transfected HEK293 cells. These results suggested that PhT2, a lysosomal membrane transporter, was up-regulated by LPS via the NF-κB signaling pathway.
Keywords: PhT2, macrophage, LPS, NF-κB, lysosome
1. Introduction
The proton-coupled oligopeptide transporter (POT) family mediates the cellular uptake of di/tripeptides and many peptidomimetics utilizing an inwardly directed proton gradient and negative membrane potential.1−3 The family consists of 4 mammalian members, PepT1 (SLC15A1), PepT2 (SLC15A2), PhT1 (SLC15A4), and PhT2 (SLC15A3). All these members are predicted to contain 12 transmembrane domains with both the N- and C-termini facing the cytosol.1,4 The majority of POT isoform characterization studies have focused on PepT1 and PepT2, which have been widely demonstrated to actively mediate the influx of numerous peptide-based therapeutic agents. They also have been shown to mediate the transport of bacterially produced peptidomimetics, like l-Ala-γ-d-Glu-meso-diaminopimelic acid and muramyl dipeptide,5,6 which may initiate nucleotide-binding oligomerization domain (NOD)-like receptor signaling pathways to induce the production of pro-inflammatory cytokines.7 In contrast, there is scant information on PhT1 and PhT2, which transport histidine in addition to di/tripeptide substrates and drugs.8
Recent studies suggest that PhT1 is associated with diabetes,9 inflammatory bowel disease,10 and systemic lupus erythematosus11 and promotes colitis through Toll-like receptor 9 and NOD1-dependent innate immune responses.12 The physiological role and pharmacologic relevance of PhT2, however, has remained largely unknown. PhT2 is predominately expressed in the spleen, thymus, and lung of rat, and its subcellular localization was identified in the lysosomes of HEK293T and BHK transfected cells.13 As the transport activity of PhT2 is pH-dependent, it is assumed to transport histidine and oligopeptide fragments, produced from digested proteins in the lysosomes, (pH ≈ 5.0) to the cytosol (pH ≈ 7.2). Therefore, it is conceivable that PhT2 may play an important role in the protein catabolic pathway where it can maintain intracellular balance.
Macrophages are key players in the immune response in which they stand sentinel against invading pathogens in the cell. Their role is to phagocytize, or engulf and then digest, cellular debris and pathogens. They also have an important role to help regulate host defense and secrete inflammatory cytokines.14 Studies by our group in macrophages isolated from mouse and human spleen,5 and that of others in macrophages generated from blood monocytes,15 demonstrated that PhT2 was highly expressed in these macrophage preparations. Therefore, we hypothesized that the physiological role of PhT2 in macrophages was to mediate the transport of protein digestion products out from lysosomes and that the functional expression of PhT2 was up-regulated when macrophages were invaded by pathogens.
To verify these hypotheses, we first studied the regulation of PhT2 macrophage expression in vitro and in vivo by pretreatment with lipopolysaccharide (LPS). LPS is a predominant, integral structural component of the outer membrane of Gram-negative bacteria and is one of the most potent microbial initiators of inflammation. It can be specifically recognized on the membrane by Toll-like receptor 4 (TLR4), which subsequently activates NF-κB and induces macrophages to produce cytokines such as TNF-α, IL-1, and IL-6.16,17 We specifically showed that the regulation of PhT2 by LPS was NF-κB mediated and that PhT2 was a lysosomal membrane protein. Our findings suggest that, in addition to transporting di/tripeptides and histidine from lysosome into cytosol, PhT2 may play an important role in immune response. This is significant since the expression of PhT2 in macrophages and mouse spleen may impact the cellular pharmacokinetics of drugs targeting these sites.
2. Materials and Methods
2.1. Materials
Primers used in qRT-PCR analyses were synthesized by Sangon Corporation (Shanghai, China). Primary antibodies used in the present study included a rabbit antirat polyclonal antibody for PhT2 (with cross reactivity for mouse PhT2),5 the mouse monoclonal antibody for β-actin from Thermo Scientific company (Rockford, IL, USA), the rabbit antihuman lysosome-associated membrane protein 1 (LAMP1) antibody from Santa Cruz Biotechnology (Dallas, TX, USA), and the anti-His Tag mouse monoclonal antibody from EarthOx (San Francisco, CA, USA). Secondary antirabbit and antimouse antibodies were obtained from Multisciences Biotech Corporation (Hangzhou, China), and FITC or Cy3-conjugated secondary antibodies were provided by Beyotime (Shanghai, China). Phorbol-12-myristate-13-acetate (PMA), lipopolysaccharides (LPS), pyrrolidine dithiocarbamate (PDTC), and other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Animals and Cell Culture
Male adult Institute of Cancer Research (ICR) mice (20 ± 2 g) were provided by the Experimental Animal Center of The Zhejiang Academy of Medicinal Sciences. Animals were allowed free access to standard laboratory chow and tap water. All procedures were approved by the institutional animal care and use committee of Zhejiang University.
The human acute monocytic leukemia cell line THP-1 and mouse macrophage cell line J774A.1 were kindly provided by Prof. Jie Yan and Prof. Dajing Xia, respectively, from the School of Medicine, Zhejiang University. Both cell lines were grown in RPMI-1640 medium supplemented with 10% fetal calf serum (Life Technologies, Grand Island, NY, USA) at 37 °C with 5% CO2. THP-1 cells were differentiated into macrophages by PMA (100 ng/mL) treatment for 48 h. LPS (10 ng/mL) and PDTC (25 μM) were used to stimulate THP-1 and J774A.1 cells. At the end of incubation, cells were harvested for qRT-PCR analyses. HEK293 cells were maintained in DMEM (Dulbecco’s Modified Eagle’s Medium) supplemented with 10% FBS (fetal bovine serum) in a humidified 5% CO2 air atmosphere at 37 °C.
2.3. qRT-PCR Assay
The mRNA expression of PhT2, IL-6, and TNFα in cells and tissues was determined by qRT-PCR using species-specific primers, as described previously.18 In brief, RNA was isolated from cells or tissues with the RNeasy Mini Kit (Qiagen GmbH, Düsseldorf, Germany). The cDNA was synthesized using 500 ng of RNA and the PrimeScriptRT reagent Kit (TaKaRa, Otsu, Japan) according to the manufacturer’s protocol. Target gene expression was determined using 2 μL cDNA with SYBR Premix Ex Taq (TaKaRa, Otsu, Japan) and primers described previously18 on the Applied Biosystems StepOne Plus Real-Time PCR system (Life Technologies, Grand Island, NY, USA). The cDNA of each sample was measured three times, and the averaged cycle threshold (CT) was used. Relative mRNA levels of target genes were normalized by GAPDH, using the ΔCT method and described as 2–ΔCT, ΔCT = av CT(target gene) – av C (GAPDH). To determine the fold-change, results were calculated using the ΔΔCT (comparative threshold cycle) method and described as 2–ΔΔCT, ΔΔCT = (av CT(target gene) – av CT(GAPDH))sample – (av CT(target gene) – av CT(GAPDH))control.
2.4. Western Blot Analysis
The protein expression of mPhT2 was evaluated in mouse tissues by Western blot analysis as described previously.5 In general, protein samples of tissues were isolated by RIPA buffer, and protein concentrations were determined with the BCA Protein Assay Kit (Beyotime, Shanghai, China). Equal amounts of total protein in the sample (∼40 μg) were separated by 10% SDS-PAGE. Protein in the gel was electroblotted onto polyvinylidene fluoride membrane (Millipore Corporation, Billerica, MA, USA) and blocked with 5.0% nonfat milk overnight in Tris-buffered saline/Tween 20 (TBST) at 4 °C. The membrane was then incubated with polyclonal antibody (PhT2, 1:500 in the blocking buffer) or monoclonal antibody (β-actin, 1:10000 in the blocking buffer) for 2 h with gentle shaking. After washing with TBST for 5 min, five times, the membrane was incubated with secondary antibody (horseradish peroxidase/conjugated goat antirabbit IgG, 1:5000 in the blocking buffer) for 1 h and then washed again five times. PhT2 and β-actin proteins were detected on X-ray film by an enhanced chemiluminescence system (ECL, LumiGlO, Gaithersburg, MD, USA). All the Western blot results were obtained under nonsaturated conditions.
2.5. Stimulation of Mice with LPS
In the LPS stimulation study, 16 male adult ICR mice were divided into four groups. Three groups of mice were administrated LPS at a dose of 1.0 mg/kg (i.p. injection), whereas the control group was administrated saline alone (i.p. injection). Mice were sacrificed by ether inhalation at designated times, and the serum was harvested for analysis of IL-6 and TNFα protein expression by ELISA. The spleen was also harvested for determination of mPhT2, IL-6, and TNFα mRNA expression by qRT-PCR. To determine if the NF-κB signaling pathway was involved in regulating PhT2, 16 mice were again divided into four groups, including the LPS group, the LPS + PDTC group, the PDTC group, and the control group (saline alone). PDTC (100 mg/kg) was administrated (i.p. injection) 1 h prior to the administration of LPS (1.0 mg/kg, i.p. injection). Mice were sacrificed after 4 h, at which time spleen samples were obtained as described above.
2.6. Transfection and Immunofluorescence
HEK293 cells were seeded in coverslips 24 h before transfection to achieve 60–80% confluence. Cells were transfected with mPhT2-His-pcDNA3.1/Hygro+ plasmids by using Lipofectamine2000 (Life Technologies, Grand Island, NY, USA) according to the manufacturer’s protocol, with 0.5 μg of DNA per well. After 24 h, cells adherent to the coverslips were fixed with 4% (w/v) para-formaldehyde in PBS for 10 min, and then treated with 0.1% Triton X-100 in PBS for 10 min. After washing with PBS three times, the cells were treated with 2% BSA in PBS for 30 min to block nonspecific protein binding. For double immunostaining, primary antibodies in 1% BSA-PBS were overlaid onto the coverslips for 45 min, the cells washed three times with PBS, and then incubated with secondary antibodies for 45 min. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). All steps were performed at room temperature. Cells were analyzed by confocal microscopy (Zeiss, Stuttgart, Germany).
2.7. Quantification of Cytokines in Serum
Serum levels of the proinflammatory cytokines IL-6 and TNF-α were quantified using a Quantikine kit (R&D Systems, Inc., Minneapolis, MN) according to manufacturer’s protocol.
2.8. Statistics
Statistical analyses were performed using GraphPad Prism version 5.01 for Windows (La Jolla, CA, USA), and scanning densitometry of the Western blots was performed using Labworks 4.0 (Upland, CA, USA). All data are given as mean ± SD. Unpaired two-tailed Student’s t test was used to compare differences between two groups. For multiple comparisons, one-way analysis of variance (ANOVA) was used followed by Dunnett’s or Tukey’s post hoc test. A probability of p ≤ 0.05 was considered significant.
3. Results
3.1. Regulation of PhT2 mRNA and Protein Expression in Macrophages by LPS
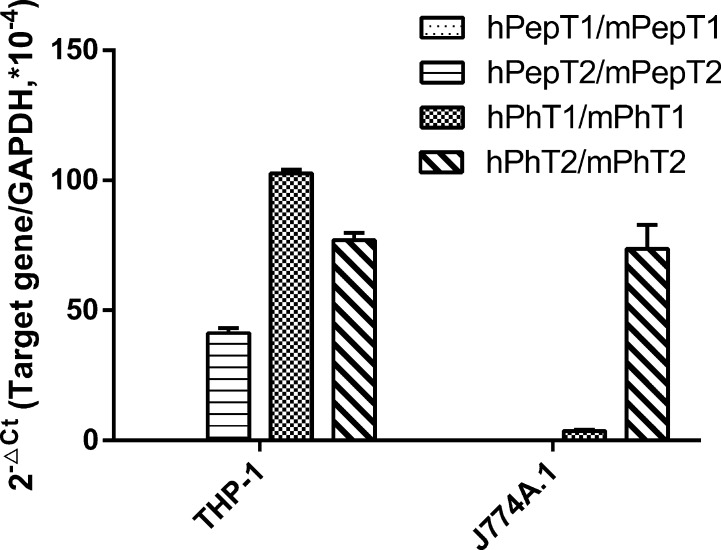
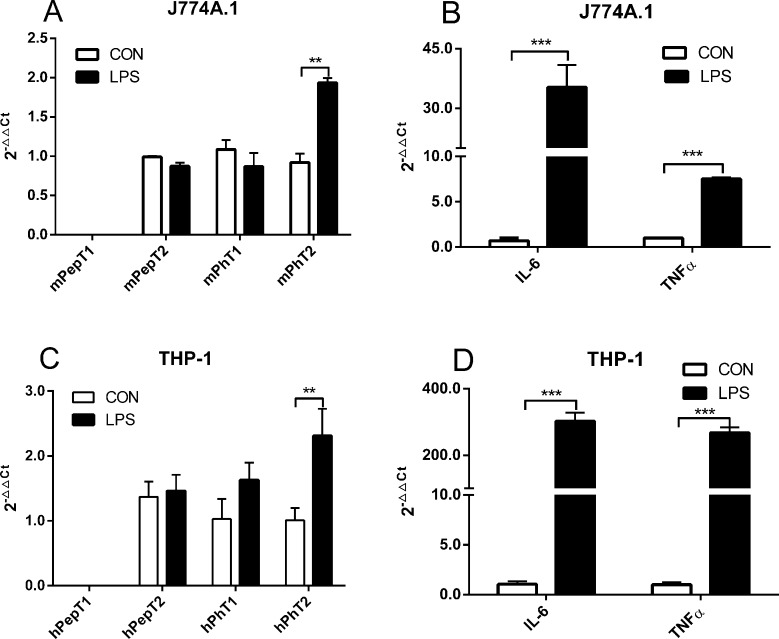
To clarify the regulation of PhT2 expression in macrophages, THP-1 and J774A.1 macrophage cell lines were chosen for the present study. The total RNA from cells was reverse-transcribed to cDNA, quantitated by qRT-PCR and then normalized by GAPDH (Figure 1). THP-1 cells (differentiated by PMA) expressed hPhT1, hPhT2, and hPepT2, with little or no expression of hPepT1. J774A.1 cells expressed mPhT2, with little or no expression of mPepT1, mPepT2, and mPhT1. To investigate the possible physiological function of POTs in macrophages, LPS (10 ng/mL), which is well-known to induce the cellular release of pro-inflammatory cytokines,19 was used to stimulate macrophages. The results revealed that PhT2 mRNA expression was increased 2-fold with LPS treatment (10 ng/mL, 2 h), while the three other POTs were not changed (Figure 2A,C). In addition, expression of the pro-inflammatory cytokines IL-6 and TNFα, two important indicators of inflammatory stimulation, was substantially increased in cells treated with LPS (Figure 2B,D).
Figure 1.
Expression of hPepT1, hPepT2, hPhT1, and hPhT2 mRNA in THP-1 cells, and the expression of mPepT1, mPepT2, mPhT1, and mPhT2 mRNA in J774A.1 cells. Data are expressed as mean ± SD, n = 3.
Figure 2.
Effect of LPS (10 ng/mL, 2 h) on the mRNA expression of POTs in J77A.1 and THP-1 macrophages. (A,C) The expression of mPepT1/hPepT1, mPepT2/hPepT2, and mPhT1/hPhT1 mRNA was not affected, whereas LPS significantly induced the expression of mPhT2/hPhT2 mRNA in J774A.1 and THP-1 macrophages. (B,D) LPS significantly induced the mRNA expression of IL-6 and TNFα in both cell lines. Data are expressed as mean ± SD, n = 3. Statistical analyses were performed by unpaired two-tailed Student’s t test. **p < 0.01 and ***p < 0.001 as compared to control. CON = control group.
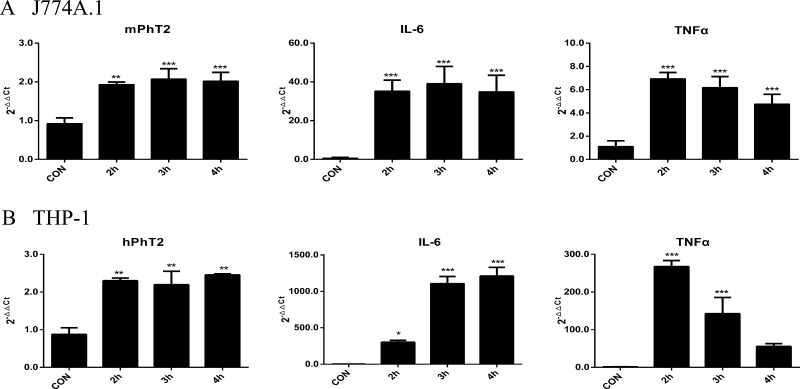
To further characterize the regulation of PhT2 expression by LPS, J774A.1 and THP-1 cells were incubated with LPS (10 ng/mL) for 2, 3, and 4 h, and the mRNA expression of PhT2 and cytokines was monitored. The results demonstrated that transcription levels of PhT2, IL-6, and TNFα were significantly up-regulated in both J774A.1 (Figure 3A) and THP-1 (Figure 3B) cells after being stimulated with LPS for 2, 3, or 4 h.
Figure 3.
Effect of LPS (10 ng/mL) induction time on the expression of mPhT2/hPhT2, IL-6, and TNFα mRNA in J77A.1 and THP-1 macrophages. In J774A.1 cells (A), mPhT2, IL-6, and TNFα mRNA were increased when treated with LPS for 2, 3, and 4 h. In THP-1 cells (B), hPhT2, IL-6, and TNFα mRNA were also increased when treated with LPS for 2, 3, and 4 h. Data are expressed as mean ± SD, n = 3. Statistical analyses were performed by one-way ANOVA and Dunnett’s test. *p < 0.05, **p < 0.01, and ***p < 0.001 as compared to control. CON = control group.
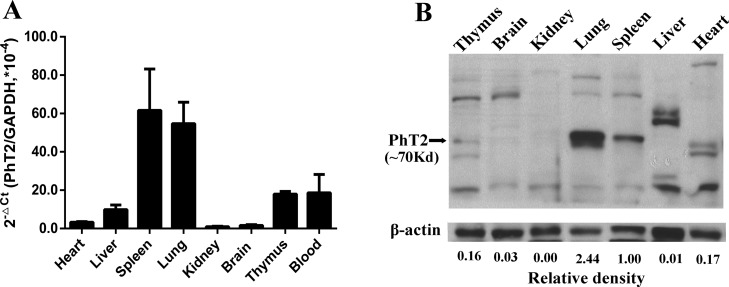
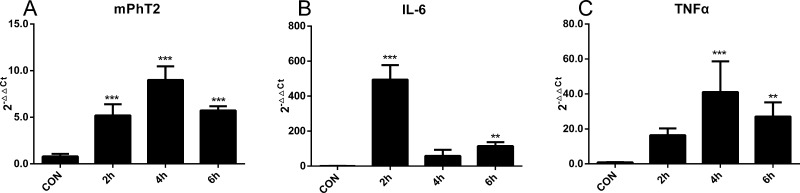
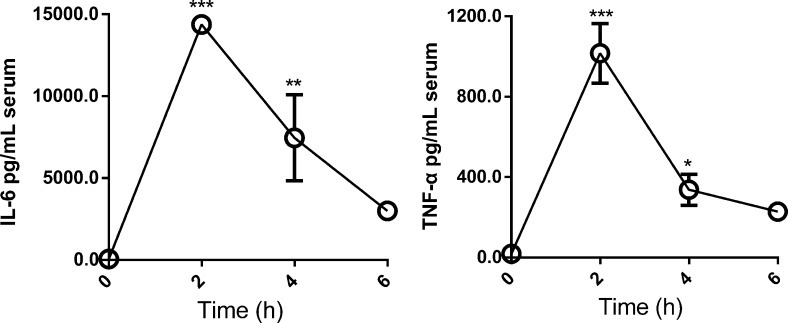
To evaluate whether PhT2 will be up-regulated by LPS in vivo, the mRNA and protein expression of mPhT2 was examined in mouse tissues. The results showed that both mPhT2 mRNA (Figure 4A) and protein (Figure 4B) were observed in lung, spleen, thymus, and heart, with the most abundant protein expression in spleen and lung. Although Figure 4B shows double bands in thymus and heart, suggesting that possible splice variants or isoforms exist, no published papers support this outcome. Moreover, the protein expression levels in these organs are low, representing <20% of that found in spleen. Since the spleen is a major organ for antibacterial immune reactivity, the regulation of mPhT2 mRNA expression in mice by LPS was studied further in this tissue. The data revealed that mPhT2 mRNA expression in mouse spleen was up-regulated 5- to 9-fold, as compared to control group, at 2, 4, and 6 h after LPS pretreatment (1.0 mg/kg) (Figure 5A). Moreover, the mRNA of IL-6 and TNFα in spleen (Figure 5B,C) and protein concentration of IL-6 and TNFα in serum (Figure 6) were significantly up-regulated, demonstrating the LPS-induced effect on inflammatory response. These in vivo results were consistent with that observed in THP-1 and J774A.1 macrophages.
Figure 4.
mRNA and protein expression of mPhT2 in mouse tissues. (A) mPhT2 mRNA was expressed abundantly in the lung and spleen, moderately in the blood, liver, and thymus, and faintly in the brain, heart, and kidney. Data are expressed as mean ± SD, n = 4. (B) mPhT2 protein was expressed abundantly in lung and spleen and mildly in heart and thymus.
Figure 5.
mRNA expression of mPhT2, TNFα, and IL-6 in mouse spleen induced by LPS. (A) mPhT2 mRNA was significantly increased in spleen when mice were injected with LPS (1.0 mg/kg i.p.) and samples obtained 2, 4, and 6 h after the injection. (B,C) IL-6 and TNFα mRNA were also up-regulated by LPS in mouse spleen. Data are expressed as mean ± SD, n = 4. Statistical analyses were performed by one-way ANOVA and Dunnett’s test. ** p < 0.01 and ***p < 0.001 as compared to control. CON = control group.
Figure 6.
Protein expression of TNFα and IL-6 in mouse serum induced by LPS. Mice were injected with LPS (1.0 mg/kg i.p.) and samples obtained 2, 4, and 6 h after the injection. TNFα and IL-6 protein was increased significantly as compared with control mice. Data are expressed as mean ± SD, n = 4. Statistical analyses were performed by one-way ANOVA and Dunnett’s test. ** p < 0.01 and ***p < 0.001 as compared to control (time zero).
3.2. NF-κB Signaling Pathway Was Involved in the Up-Regulation of PhT2 by LPS
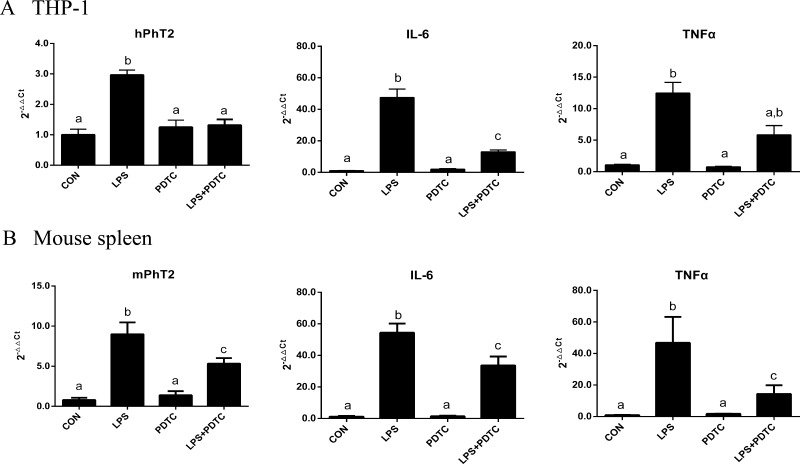
NF-κB is a nuclear factor that initiates the transcription of numerous pro-inflammatory mediators like IL-6 and TNFα. Since PhT2 and the pro-inflammatory cytokines IL-6 and TNFα had similar expression patterns (in vitro and in vivo) following LPS, we hypothesized that the NF-κB signaling pathway was involved in regulating PhT2 transcription. In order to verify this hypothesis, PDTC (an inhibitor of NF-κB) was studied with LPS in THP-1 cells. Specifically, cells were incubated with LPS (0 and 10 ng/mL) for 3 h after being pretreated with or without PDTC (0 and 25 μM) for 1 h. The results showed that PDTC alone did not affect the expression of hPhT2 but abolished the up-regulation of hPhT2 induced by LPS (Figure 7A). Similar results were observed for mPhT2 in mouse spleen (obtained 4 h after the LPS injection) using an acute inflammatory model where animals were administered LPS (0 and 1.0 mg/kg) after the 1 h pretreatment of PDTC (0 and 100 mg/kg) (Figure 7B). These spleen samples further revealed that the increased expression of IL-6 and TNFα induced by LPS was significantly reduced by the inhibition of NF-κB by PDTC (Figure 7A,B), a finding that was consistent with other studies.20,21 On the basis of these results, we deduced that NF-κB was an important factor that regulated the transcription of PhT2 in macrophages after LPS stimulation.
Figure 7.
Effect of PDTC on the mRNA up-regulation of hPhT2/mPhT2 by LPS in THP-1 macrophages (n = 3) and mouse spleen (n = 4). THP-1 macrophages (A) were treated with LPS (10 ng/mL) for 3 h following pretreatment with PDTC (25 μM) for 1 h. PDTC suppressed the mRNA up-regulation of hPhT2, IL-6, and TNFα. ICR mice (B) were injected with LPS (1.0 mg/kg i.p.) 1 h after being pretreated with PDTC (100 mg/kg, i.p.). Samples were obtained 4 h after the LPS injection. PDTC suppressed the mRNA up-regulation of mPhT2, IL-6, and TNFα. Data are expressed by mean ± SD. Groups with different letters were statistically different as determined by one-way ANOVA and Tukey’s test.
3.3. Subcellular Localization of PhT2
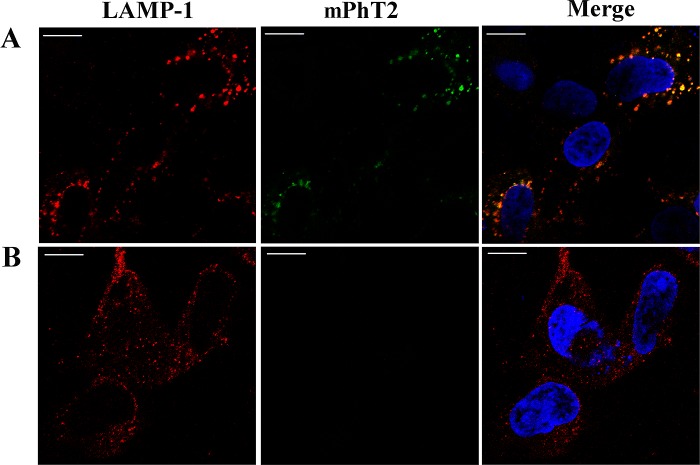
The previous results of PhT2 expression and function in macrophages motivated us to confirm its subcellular localization. PhT2 possesses a dileucine-based motif in the N-terminal region, which is a signal for sorting to lysosomes. Thus, we developed a mouse PhT2 construct containing a C-terminally appended 6× His-Tag (mPhT2-His-pcDNA3.1(+)) plasmid where anti-His and anti-LAMP1 antibodies were used to determine the localization of mPhT2 protein. HEK293 cells were transiently transfected with mPhT2-His-pcDNA3.1(+) plasmids for 24 h. Immunofluorescence and confocal microscopy were then performed, and the results showed that mPhT2 proteins were colocalizing with LAMP1, a marker of lysosomes (Figure 8). Our results provided strongly supportive evidence that mPhT2 was localized on the lysosomal membrane. We further hypothesize that PhT2 may function to transport di/tripeptides and/or histidine from lysosomes into the cytosol, as has been inferred previously.13 Importantly, subcellular localization studies will need to be performed in macrophages and mouse tissues to confirm the lysosomal relevance of PhT2 in native tissue.
Figure 8.
Expression of mPhT2 protein in lysosomes. At 24 h post-transfection, HEK293 cells were fixed, permeabilized, and incubated with antibodies against His-Tag and LAMP1. Primary antibodies were detected with FITC and Cy3-conjugated secondary antibodies. (A) HEK293 cells were transiently transfected with mPhT2-His-pcDNA3.1(+). The overlay (yellow) reveals the colocalization of mPhT2 (green) with LAMP1 (red). (B) HEK293 cells were transiently transfected with empty pcDNA3.1(+) vector. Nuclei (blue) were stained with DAPI. Bar = 10 μm.
4. Discussion
The results presented here established that PhT2 is expressed on lysosomal membranes and that it can be significantly induced in macrophages by LPS. This up-regulation was likely mediated by the NF-κB signaling pathway. Macrophages play an important role in antigen processing, defense against microorganisms, and various inflammatory processes. In the present study, we found that PepT2, PhT1, and PhT2, but not PepT1, transcripts were expressed in macrophages, with PhT2 having the greatest expression compared to other members of the POT family. Interestingly, when human or mouse macrophages were treated with LPS, only PhT2 transcripts were up-regulated in a significant manner. The expression and function of POTs are regulated by a variety of factors, such as hormones, pathological state, and cytokines. Inflammatory bowel disease and diet also have distinct regulatory effects on PepT1.22−26 Moreover, epidermal growth factor and intracellular Ca2+ concentration can affect the expression and function of PepT2.27,28 However, limited information is available on the regulation or mechanism of regulation for PhT1 and PhT2. Our study revealed that LPS could up-regulate the expression of PhT2. Moreover, the mRNA and protein expression of PhT2 was mainly observed in mouse spleen and lung. The totality of these results suggested that PhT2 may play a special role in the ability of macrophages to react to immune response and raises the possibility that PhT2 may represent a new cellular target for drugs and drug delivery strategies. This finding also prompted us to further study the mechanism concerning the up-regulation of PhT2 in macrophages after LPS stimulation.
LPS can be recognized by TLR4 on macrophage membranes and activate the downstream NF-κB signaling pathway. NF-κB is a prominent transcription factor suggested to be a central regulator of genes and end-effectors of the host’s inflammatory response.29 The NF-κB family has five members, including RelA (p65), RelB, and c-Rel, and the precursor proteins NF-κB1 (p105) and NF-κB2 (p100), which are processed into p50 and p52, respectively.30 In resting cells, NF-κB is retained as an inactive complex bound to the cytoplasmic inhibitor protein IκBα. When cells are stimulated with LPS, phosphorylation and degradation of IκBα permit the NF-κB complex to translocate to the nucleus and effect gene transcription.31 NF-κB’s target genes include cytokines, chemokines, major histocompatibility complex molecules, proteins involved in antigen presentation, and receptors required for neutrophil adhesion and transmigration across blood vessel walls.32 In our study, we demonstrated that LPS activated NF-κB in macrophages to induce the expression of PhT2. Using the software PROMO,100 we found several putative transcription factor binding sites of NF-κB on the promoter sequence of PhT2. In order to confirm the importance of NF-κB in the up-regulation of PhT2 by LPS, we used PDTC to conduct both in vitro and in vivo inhibition experiments. PDTC is commonly used as a selective NF-κB inhibitor as it prevents both the degradation of IκBα and the translocation of NF-κB complex to the nucleus.21,33 When macrophages or mice were pretreated with PDTC, we observed the NF-κB-mediated stimulation of PhT2 by LPS to be significantly suppressed. Likewise, the cytokines IL-6 and TNFα, chosen as positive controls for LPS-induced inflammation, were suppressed in macrophages by PDTC in a similar manner to that of PhT2 after LPS induction. Still, the exact binding site of NF-κB on the promoter sequence of PhT2 needs further study.
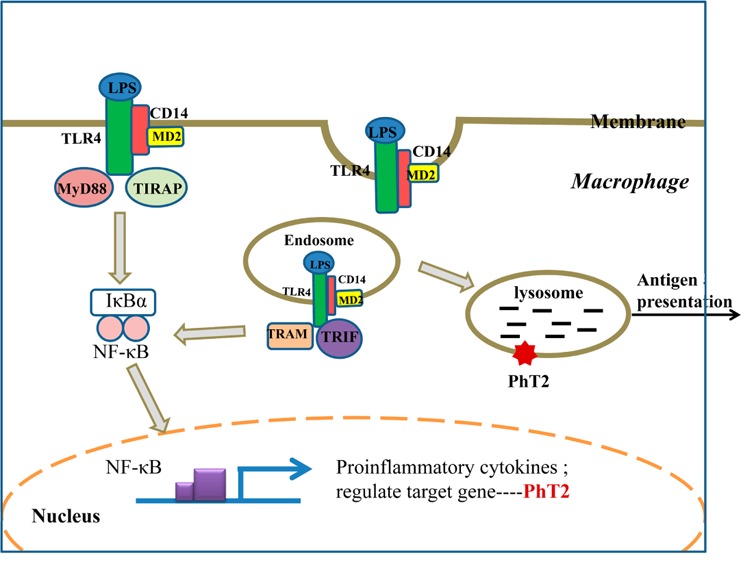
PhT2 has high sequence similarity with PhT1, and both PhT1/2 have a dileucine-based motif in the N-terminal region that provides a signal for sorting to lysosomes.34 A previous study13 and our current study indicate that PhT2 is a membrane protein localized on lysosomes. Since lysosomes are digestive organelles containing enzymes that operate best at pH ≈ 5.0 to yield amino acid and small peptide digestion products, the intracellular localization of PhT2 in macrophages is logical. LPS induces assembly of a ligand-binding complex consisting of CD14 (pattern recognition receptor), MD-2, and TLR4 at the plasma membrane,35 in which the complex induces two distinct signaling pathways controlled by the adaptor proteins TIRAP-MyD88 and TRAM-TRIF. Specifically, the complex induces TIRAP-MyD88 signaling at the plasma membrane to active NF-κB. The complex is also endocytosed and activates TRAM-TRIF signaling from early endosomes.36 These endosomes can fuse with lysosomes to effect degradation of the LPS complex, which then helps present antigens to the CD4+ T helper cells.37 With the increase of LPS complex and other intracellular products in macrophages stimulated by LPS, the lysosomes will need to work harder to degrade and recycle endosomal protein degradation products and thereby maintain intracellular homeostasis. In doing so, the up-regulation of PhT2 on lysosomal membranes will be needed to transport the additionally formed di/tripeptides and histidine from lysosomes into the cytosol. A possible model for the LPS-induced up-regulation of signaling pathways and PhT2 in macrophage is shown in Figure 9.
Figure 9.
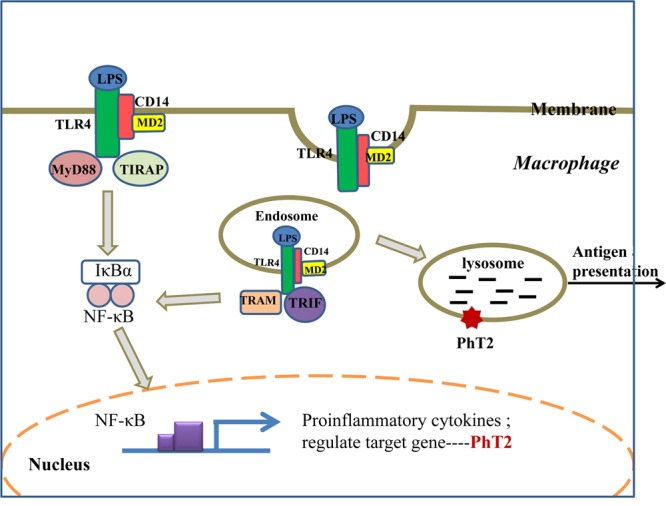
LPS up-regulates the PhT2 signaling pathway. LPS is specifically recognized by Toll-like receptor 4 (TLR4) on the membrane to form a complex consisting of CD14, TLR4, and MD2, which signals TIRAP-MyD88 adaptors to activate NF-κB and up-regulate the expression of inflammatory cytokines and PhT2. The LPS complex is also endocytosed through one or more dynamin-dependent pathways. In endosomes, a second phase of TRAM-TRIF-mediated signaling is initiated, which leads to further NF-κB signaling and the production of inflammatory cytokines and PhT2. Endosomes are fused with lysosomes for degradation of the LPS complex, which utilizes transporters on the lysosomal membrane to help antigen presentation.
The increasing importance of PepT1 and PepT2 in drug delivery, targeting, and pharmacological response is becoming more evident.1 However, little is known about the expression, function, and regulation of PhT2 especially in macrophage. It is particularly intriguing that PhT2 may be associated with the immune response. Thus, new information regarding an elusive POT may reveal a new cellular target for various drugs such as antibiotics, antiviral drugs, and anti-inflammatory agents. It may also be important to develop new drug delivery strategies for efficient targeting. Nevertheless, the precise mechanism of PhT2-mediated di/tripeptide and histidine transport at the lysosomal membrane remains to be determined, along with PhT2’s substrate specificity and transport kinetics.
In summary, the present study provides important evidence of PhT2 expression in mouse tissues and macrophage cell lines. Furthermore, the study demonstrated that LPS was a distinct positive regulatory factor of PhT2 expression and that this regulation occurred mainly through the NF-κB signaling pathway. The intracellular localization of PhT2, which turns out to be a lysosomal membrane protein, suggests this POT family member might play an important role in immune response.
Acknowledgments
We gratefully acknowledge Prof. Dajing Xia and Prof. Jie Yan for kindly providing us with the THP-1 and J774A.1 cells. This work was supported by Natural Scientific Foundation of China (81072689), by National Major Projects for Science and Technology Development of Ministry Science and Technology of China (2012ZX09506001-004), and by NIH Grant R01-GM035498 (to D.E.S.).
Glossary
Abbreviations Used
- POTs
proton-coupled oligopeptide transporters
- LPS
lipopolysaccharide
- PDTC
pyrrolidine dithiocarbamate
- qRT-PCR
quantitative real-time PCR
- LAMP1
lysosome-associated membrane protein 1
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- IL-6
interleukin-6
- TNFα
tumor necrosis factor alpha
- NOD
nucleotide-binding oligomerization domain
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Smith D. E.; Clemencon B.; Hediger M. A. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol. Aspects Med. 2013, 342–3323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Aliaga I.; Daniel H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica 2008, 387–81022–42. [DOI] [PubMed] [Google Scholar]
- Brandsch M.; Knutter I.; Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. J. Pharm. Pharmacol. 2008, 605543–85. [DOI] [PubMed] [Google Scholar]
- Nielsen C. U.; Brodin B. Di/tri-peptide transporters as drug delivery targets: Regulation of transport under physiological and patho-physiological conditions. Curr. Drug Targets 2003, 45373–388. [DOI] [PubMed] [Google Scholar]
- Sun D.; Wang Y.; Tan F.; Fang D.; Hu Y.; Smith D. E.; Jiang H. Functional and molecular expression of the proton-coupled oligopeptide transporters in spleen and macrophages from mouse and human. Mol. Pharmaceutics 2013, 1041409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso G.; Nguyen H. T. T.; Charrier-Hisamuddin L.; Yan Y.; Laroui H.; Demoulin B.; Sitaraman S. V.; Merlin D. PepT1 mediates transport of the pro-inflammatory bacterial tripeptide l-Ala-gamma-d-Glu-meso-DAP in intestinal epithelial cells. Am. J. Physiol.: Gastrointest. Liver Physiol. 2010, 2993G687–G696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W.; Murray P. J.; Kitani A.; Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 2006, 619–20. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R. K.; Herrera-Ruiz D.; Eltoukhy N.; Saad M.; Knipp G. T. The functional evaluation of human peptide/histidine transporter 1 (hPHT1) in transiently transfected COS-7 cells. Eur. J. Pharm. Sci. 2006, 275533–542. [DOI] [PubMed] [Google Scholar]
- Takeuchi F.; Ochiai Y.; Serizawa M.; Yanai K.; Kuzuya N.; Kajio H.; Honjo S.; Takeda N.; Kaburagi Y.; Yasuda K.; Shirasawa S.; Sasazuki T.; Kato N. Search for type 2 diabetes susceptibility genes on chromosomes 1q, 3q and 12q. J. Hum. Genet. 2008, 534314–324. [DOI] [PubMed] [Google Scholar]
- Lee J.; Tattoli I.; Wojtal K. A.; Vavricka S. R.; Philpott D. J.; Girardin S. E. pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling. J. Biol. Chem. 2009, 2843523818–23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C. F.; Liu Y. S.; Cheng Y. L.; Gao J. P.; Pan T. M.; Han J. W.; Quan C.; Sun L. D.; Zheng H. F.; Zuo X. B.; Xu S. X.; Sheng Y. J.; Yao S.; Hu W. L.; Li Y.; Yu Z. Y.; Yin X. Y.; Zhang X. J.; Cui Y.; Yang S. TNIP1, SLC15A4, ETS1, RasGRP3 and IKZF1 are associated with clinical features of systemic lupus erythematosus in a Chinese Han population. Lupus 2010, 19101181–1186. [DOI] [PubMed] [Google Scholar]
- Sasawatari S.; Okamura T.; Kasumi E.; Tanaka-Furuyama K.; Yanobu-Takanashi R.; Shirasawa S.; Kato N.; Toyama-Sorimachi N. The solute carrier family 15A4 regulates TLR9 and NOD1 functions in the innate immune system and promotes colitis in mice. Gastroenterology 2011, 14051513–25. [DOI] [PubMed] [Google Scholar]
- Sakata K.; Yamashita T.; Maeda M.; Moriyama Y.; Shimada S.; Tohyama M. Cloning of a lymphatic peptide/histidine transporter. Biochem. J. 2001, 356Pt 153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin D. L. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem. Res. Toxicol. 2009, 2281376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau A.; Le Vee M.; Jouan E.; Parmentier Y.; Fardel O. Drug transporter expression in human macrophages. Fundam. Clin. Pharmacol. 2011, 256743–52. [DOI] [PubMed] [Google Scholar]
- Takeda K.; Akira S. TLR signaling pathways. Semin. Immunol. 2004, 1613–9. [DOI] [PubMed] [Google Scholar]
- Muller J. M.; Zieglerheitbrock H. W. L.; Baeuerle P. A. Nuclear factor kappa-B, a mediator of lipopolysaccharide effects. Immunobiology 1993, 1873–5233–256. [DOI] [PubMed] [Google Scholar]
- Sun D. L.; Tan F. Q.; Fang D. B.; Wang Y. Q.; Zeng S.; Jiang H. D. Expression of proton-coupled oligopeptide transporter (POTs) in prostate of mice and patients with benign prostatic hyperplasia (BPH) and prostate cancer (PCa). Prostate 2013, 733287–295. [DOI] [PubMed] [Google Scholar]
- Tynan R. J.; Weidenhofer J.; Hinwood M.; Cairns M. J.; Day T. A.; Walker F. R. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain, Behav., Immun. 2012, 263469–479. [DOI] [PubMed] [Google Scholar]
- Ohta K.; Nakayama K.; Kurokawa T.; Kikuchi T.; Yoshimura N. Inhibitory effects of pyrrolidine dithiocarbamate on endotoxin-induced uveitis in Lewis rats. Invest. Ophthalmol. Visual Sci. 2002, 433744–50. [PubMed] [Google Scholar]
- Liu S. F.; Ye X. B.; Malik A. B. Inhibition of NF-kappa B activation by pyrrolidine dithiocarbamate prevents in vivo expression of pro-inflammatory genes. Circulation 1999, 100121330–1337. [DOI] [PubMed] [Google Scholar]
- Foster D. R.; Landowski C. P.; Zheng X.; Amidon G. L.; Welage L. S. Interferon-gamma increases expression of the di/tri-peptide transporter, h-PEPT1, and dipeptide transport in cultured human intestinal monolayers. Pharmacol. Res. 2009, 593215–20. [DOI] [PubMed] [Google Scholar]
- Radeva G.; Buyse M.; Hindlet P.; Beaufils B.; Walker F.; Bado A.; Farinotti R. Regulation of the oligopeptide transporter, PEPT-1, in DSS-induced rat colitis. Dig. Dis. Sci. 2007, 5271653–61. [DOI] [PubMed] [Google Scholar]
- Ashida K.; Katsura T.; Motohashi H.; Saito H.; Inui K. Thyroid hormone regulates the activity and expression of the peptide transporter PEPT1 in Caco-2 cells. Am. J. Physiol.: Gastrointest. Liver Physiol. 2002, 2824G617–23. [DOI] [PubMed] [Google Scholar]
- Nielsen C. U.; Amstrup J.; Steffansen B.; Frokjaer S.; Brodin B. Epidermal growth factor inhibits glycylsarcosine transport and hPepT1 expression in a human intestinal cell line. Am. J. Physiol.: Gastrointest. Liver Physiol. 2001, 2811G191–9. [DOI] [PubMed] [Google Scholar]
- Thamotharan M.; Bawani S. Z.; Zhou X.; Adibi S. A. Functional and molecular expression of intestinal oligopeptide transporter (Pept-1) after a brief fast. Metabolism 1999, 486681–4. [DOI] [PubMed] [Google Scholar]
- Bravo S. A.; Nielsen C. U.; Amstrup J.; Frokjaer S.; Brodin B. Epidermal growth factor decreases PEPT2 transport capacity and expression in the rat kidney proximal tubule cell line SKPT0193 cl.2. Am. J. Physiol.: Renal, Fluid Electrolyte Physiol. 2004, 2862F385–F393. [DOI] [PubMed] [Google Scholar]
- Wenzel U.; Diehl D.; Herget M.; Kuntz S.; Daniel H. Regulation of the high-affinity H+/peptide cotransporter in renal LLC-PK1 cells. J. Cell. Physiol. 1999, 1783341–348. [DOI] [PubMed] [Google Scholar]
- Baetz D.; Shaw J.; Kirshenbaum L. A. Nuclear factor-kappaB decoys suppress endotoxin-induced lung injury. Mol. Pharmacol. 2005, 674977–9. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus A.; Hayden M. S.; Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat. Immunol. 2011, 128695–708. [DOI] [PubMed] [Google Scholar]
- Hayden M. S.; Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004, 18182195–224. [DOI] [PubMed] [Google Scholar]
- Takeda K.; Akira S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 1711–14. [DOI] [PubMed] [Google Scholar]
- PROMO. http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3.
- Zieglerheitbrock H. W. L.; Sternsdorf T.; Liese J.; Belohradsky B.; Weber C.; Wedel A.; Schreck R.; Bauerle P.; Strobel M. Pyrrolidine dithiocarbamate inhibits Nf-kappa-B mobilization and Tnf production in human monocytes. J. Immunol. 1993, 151126986–6993. [PubMed] [Google Scholar]
- Haft C. R.; Klausner R. D.; Taylor S. I. Involvement of dileucine motifs in the internalization and degradation of the insulin receptor. J. Biol. Chem. 1994, 2694226286–94. [PubMed] [Google Scholar]
- Zanoni I.; Ostuni R.; Marek L. R.; Barresi S.; Barbalat R.; Barton G. M.; Granucci F.; Kagan J. C. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 2011, 1474868–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. C.; Su T.; Horng T.; Chow A.; Akira S.; Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat. Immunol. 2008, 94361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husebye H.; Halaas O.; Stenmark H.; Tunheim G.; Sandanger O.; Bogen B.; Brech A.; Latz E.; Espevik T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006, 254683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]