Figure 3.
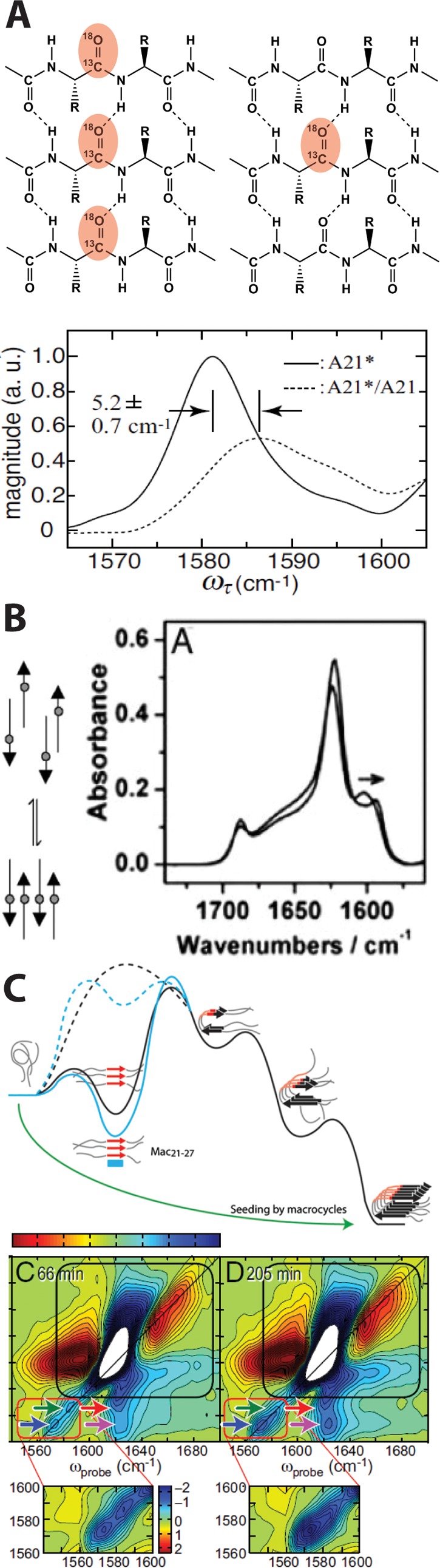
Effect of vibrational coupling in the IR spectra of 13C=18O amyloids. (A) Parallel in-register β-sheets with each strand containing identical 13C=18O labels (left, red) and dilute 13C=18O labels (right, red). In-register labeling and isotope dilution of the amyloid peptide Aβ (bottom) demonstrated the low-frequency fully coupled labeled amide I mode and the blue shift in label frequency that occurs upon dilution with 12C=16O residues. The lower panel is reproduced from ref (38), copyright 2008, Proceedings of the National Academy of Sciences of America. (B) Equilibration of amyloid aggregates from out-of-register to stable in-register species is accompanied by a shift in the 13C=18O label frequency as labels (gray circles) align. Reproduced from ref (46), copyright 2005, Proceedings of the National Academy of Sciences of America. (C) 13C=18O-labeled variants of hIAPP define the order of amino acid incorporation into amyloid β-sheets via a shift in label frequency and intensity and the appearance of a cross-peak between labeled and unlabeled β-sheets in the 2D IR spectra. A transient β-sheet intermediate (top panel) was identified based on the appearance of linearly coupled labels prior to stable fiber formation. Reproduced from refs (28) and (47), copyright 2013 and 2009, respectively, Proceedings of the National Academy of Sciences of America.
