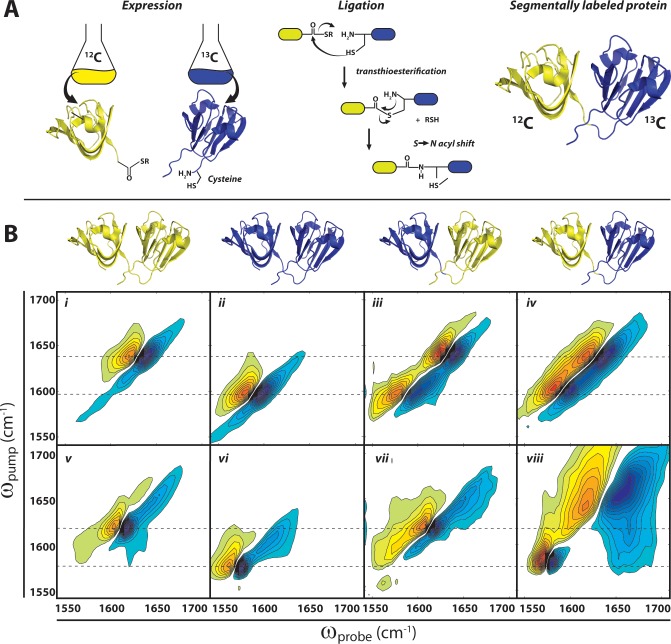
Figure 5.
Segmental 13C labeling of γD-crystallin. (A) Use of expressed protein ligation to generate segmentally labeled proteins. Individual domains are expressed separately in E. coli with reactive termini (C-terminal thioester and N-terminal cysteine), and subsequent transthioesterification and peptide bond formation via an S → N acyl shift generates the full-length protein with one isotope-labeled domain. (B) 2D IR spectra of γD-crystallin. (Top row) Native structures showing the location of 12C (yellow) and 13C (blue) domains in unlabeled (12C), uniformly 13C-labeled, C-terminally labeled, and N-terminally 13C-labeled γD-crystallin. (Middle row) (i–iv) 2D IR spectra of native γD-crystallin labeling variants. (Bottom row) (v–viii) 2D IR spectra of acid-induced amyloid fibers of γD-crystallin labeling variants. All 2D IR spectra are plotted based on data first reported in ref (19). Dashed lines indicate ωpump for labeled and unlabeled native β-sheets (i–iv) and amyloid β-sheets (v–viii).