Figure 1.
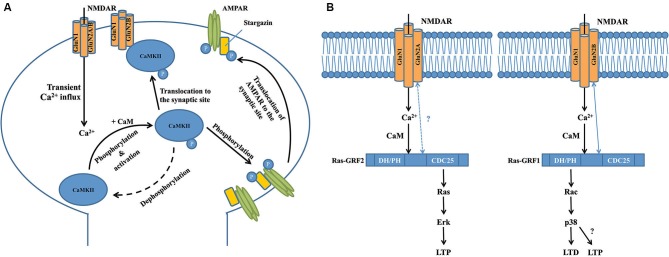
Interactions between calcium-sensing proteins and NMDARs in the NMDAR complex and their critical roles in NMDAR-dependent synaptic plasticity. (A) GluN2B-CAMKII interaction is required for the induction of long-term potentiation at the excitatory glutermaterigic synapse. At the postsynaptic domain, transient Ca2+ influx through the NMDAR induces autophosphorylation (at T286 on CAMKIIα subunits or T287 on CAMKIIβ subunits) of CaMKII, resulting in its persistent activation and subsequent translocation to the synaptic site where it binds to the GluN2B subunit of NMDAR. Activated CaMKII can phosphorylate the S831 residue of GluA1 subunit of AMPAR that significantly increases the single-channel conductance of the receptor. Meanwhile, activated CaMKII can also phosphorylate the postsynaptic scaffolding protein stargazin to facilitate the trafficking of AMPAR from the extrasynaptic space to the synaptic region so as to enhance synaptic transmission. (B) Transient calcium influx through GluN2B-containing NMDAR selectively activates Ras-GRF1 that contributes to LTD by activating the downstream Rac/p38 pathway, while calcium influx through GluN2A-containing NMDAR selectively activates Ras-GRF2 that contributes to LTP by activating the downstream Ras/ERK pathway. There is a selective physical interaction between Ras-GRF1 and the GluN2B subunit of NMDAR, but there is no evidence supporting the interaction between Ras-GRF2 and GluN2A subunit of NMDAR. Interestingly, a recent study showed that starting at 2 months of age in mice, Ras-GRF1 starts to contribute to the induction of LTP in the CA1 of hippocampus via the Rac/p38 pathway, however the exact mechanism is still unknown.
