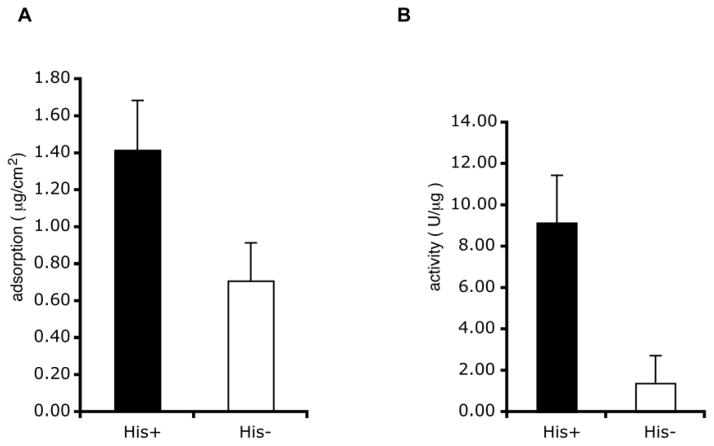
Figure 4.
Use of the amino terminal His-tag: Ni-NTA attachment chemistry improved binding and the specific activity of the GPI relative to GPI randomly adsorbed to the surface. The His-tag was cleaved from His-GPI by means of an enterokinase site provided by the vector. (A) Approximately twice as much His-GPI bound to a Ni-NTA support in comparison with control GPI lacking the His-tag (p=0.012, paired student T test; n = 9 samples, with 3 samples from each of 3 protein preparations. Bars indicate the standard deviations.). (B) Normalizing for the amount of protein bound allowed a quantification of the specific activities of the adsorbed proteins. The specific activity of the His-GPI was approximately 9 times greater than that of the randomly absorbed GPI, although the enzyme activities in solution were relatively equal (p=0.006, paired student T test; n = 9 samples, with 3 samples from each of 3 protein preparations. Bars indicate the standard deviations.).