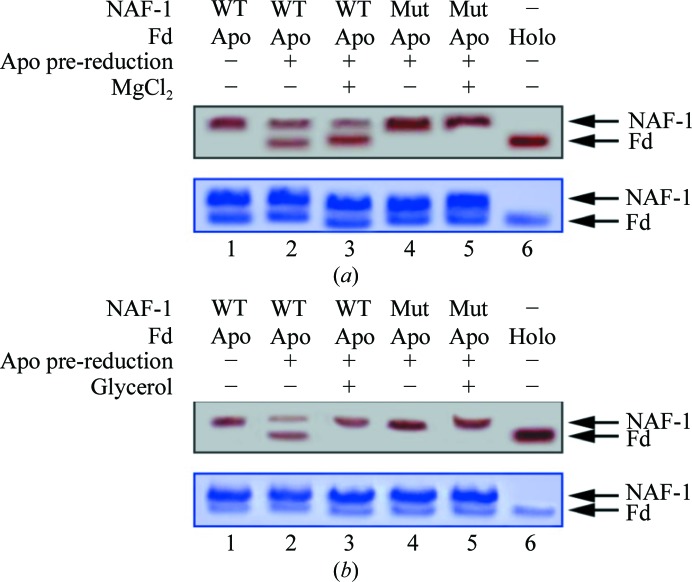
Figure 5.
The NAF-1 [2Fe–2S] cluster-transfer function is abrogated in NAF-1 H114C. NAF-1 was incubated at 37°C for 20 min with β-mercaptoethanol pre-reduced apo-Fd and run on a native gel. In the upper native gels in each panel the red-colored bands are indicative of the [2Fe–2S] cluster in the two proteins; the upper red band represents NAF-1 (labeled NAF-1 on the right side of the gel) and the lower red band represents holo-Fd (labeled Fd). (a) Cluster transfer from NAF-1 to the pre-reduced apo-Fd in the absence and presence of MgCl2. The native (WT) NAF-1 (lane 1) indicated by the red-colored upper band decreases in intensity upon incubation with pre-reduced apo-Fd, with a concomitant increase in the intensity of the lower red band owing to the formation of holo-Fd (lane 2). In the presence of MgCl2, the cluster transfer from NAF-1 to apo-Fd is slightly greater (lane 3). NAF-1 H114C completely lost the cluster-transfer function in the absence (lane 4) or presence (lane 5) of MgCl2. Lane 6 contains holo-Fd for reference. The lower gel was stained with Coomassie Blue to confirm that the protein levels were the same in all experiments. (b) Cluster transfer in the presence of glycerol. The native NAF-1 (WT; lane 1), when incubated with pre-reduced apo-Fd, transfers its cluster to the latter to form holo-Fd (lower red band, lane 2). The presence of glycerol prevents cluster transfer from NAF-1 to pre-reduced apo-Fd (lane 3). NAF-1 H114C does not function as a cluster-donor protein in the absence (lane 4) or the presence of glycerol (lane 5). Lane 6 contains the control holo-Fd.