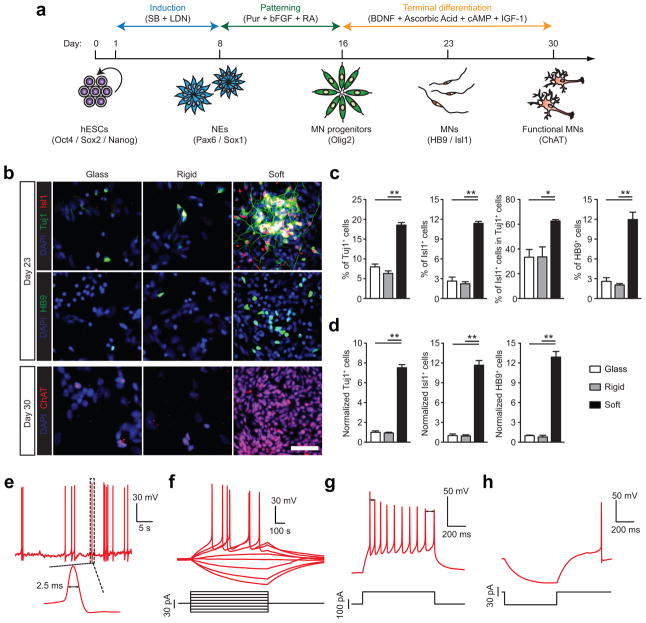
Figure 2.
Purity and yield of functional motor neurons (MNs) derived from hESCs are improved on soft substrates. (a) Schematic diagram showing experimental design for sequential neural induction, patterning, and functional maturation of MNs from hESCs. hESCs were cultured on vitronectin-coated coverslips and rigid (E = 1,200 kPa) and soft (E = 5 kPa) PMAs in neural induction medium containing the dual Smad inhibitors SB and LDN for 8 d and then in MN differentiation medium containing purmorphamine (Pur), basic fibroblast growth factor (bFGF), and retinoic acid (RA) for an additional 8 d. Putative MN progenitor cells collected at day 16 were transferred onto coverslips and cultured in MN maturation medium containing brain-derived neurotrophic factor (BDNF), ascorbic acid, cyclic adenosine monophosphate (cAMP), and insulin-like growth factor 1 (IGF-1) for another 14 d. (b) Representative immunofluorescence images showing Tuj1+, Isl1+, and HB9+ cells at day 23 (top), and ChAT+ cells at day 30 (bottom). Scale bar, 100 μm. (c&d) Bar plots showing percentages (c) and relative numbers (d) of Tuj1+, Isl1+, and HB9+ cells at day 23 as a function of substrate rigidity. Data in d was normalized to values from coverslips. Data represents the mean ± s.e.m with n ≥3. P-values were calculated using two-side unpaired student t-tests. *: P < 0.05; **: P < 0.01. (e–h) Electrophysiological characterization of functional MNs derived from soft PMAs at day 30 using whole-cell patch clamp. e: spontaneous action potential (AP) with resting membrane potential of −64.5 mV; f: voltage response to current step injection; g: instantaneous frequency change or spike frequency adaptation (SFA) evoked with positive current injection; h: post-inhibitory rebound (PIR) after hyperpolarizing current injection.