Fig. 3.
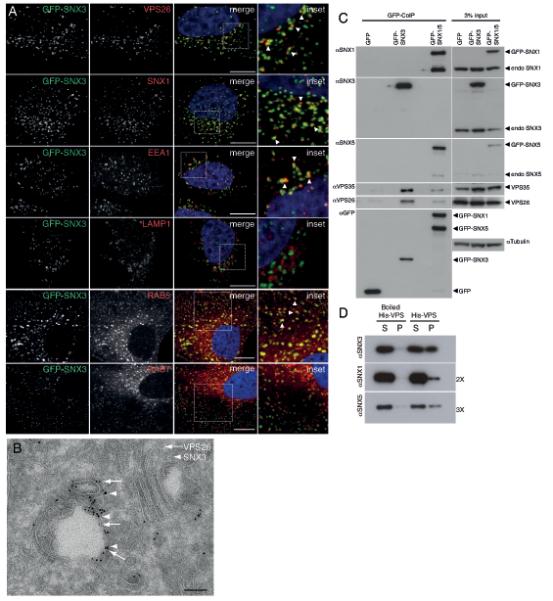
Co-localization and physical interaction of SNX3 with the cargo-selective sub-complex of the retromer. (A) SNX3 partially co-localizes with VPS26-positive early endosomes. HeLa cells lentivirally transduced to express GFP-SNX3 (green) were fixed and stained for VPS26, SNX1, EEA1 or LAMP1 (red). Co-localization between GFP-SNX3 and VPS26, SNX1, EEA1, LAMP1, Rab5 and Rab7 was quantified as 0.43 ± 0.05, 0.55 ± 0.04, 0.38 ± 0.02 and 0.07 ± 0.04, 0.61 ± 0.02, and 0.34 ± 0.02, respectively (Pearson’s coefficient, mean ± SD, n=3 with 30 cells per condition, for Rab5 and Rab7, n=20 cells). Scale bar, 11 μm. (B) At the ultrastructural level, SNX3 and VPS26 localize to common vesicular endosomal profiles. GFP-SNX3 is labeled with 10 nm gold and mCherry-VPS26 with 6 nm gold. The image is representative of that observed from the analysis of 5 other endosomal vacuoles. Scale bar, 100 nm. (C) SNX3 interacts with the cargo-selective sub-complex of the retromer. Cell extracts derived from HeLa cells lentivirally transduced with GFP, GFP-SNX3 or both GFP-SNX1 and GFP-SNX5 (GFP-SNX1/5), were subjected to GFP-nanotrap. The classical retromer SNX-BARs form heterodimeric complexes leading to the presence of both endogenous SNX1 and SNX5 in the GFP-SNX1/5 IPs3. (D) 3xFLAG-VPS26-VPS29-VPS35-His6 complex (His-VPS) was isolated from BL21 E. coli onto TALON resin and incubated with 2 μM of recombinant SNX3, SNX1 or SNX5 for 2 hours at 4°C. Supernatant (S) and TALON containing resin (P) were isolated. SNX3 directly associates with His-VPS as do SNX1 and SNX5 although this is less well pronounced (longer exposures are shown). Control: boiled His-VPS resin.
