Figure 1.
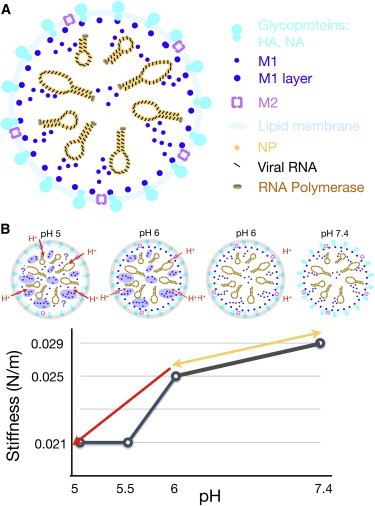
How pH impacts on Influenza virus stiffness. (A) Schematic representation of major structural features of the Influenza A virus, including the lipid envelope with the glycoproteins hemagglutinin (HA) and neuraminidase (NA), the M2 channel, the matrix protein M1, and the viral genome consisting of eight ribonucleoprotein particles made up of the nucleoprotein (NP) and negative-stranded RNA. (B) Two-step pH-dependent softening of IAV. (Upper part of panel) Changes in the virus are represented by four schematic drawings. (Lower part of panel) Simplified stiffness plot as a function of pH (taken from Li et al. (38)). The conversion from the neutral to the pH 6 form of IAV is reversible, and first leads to a softening of the glycoprotein layer (represented by shaded circles in the schematic figure). A small amount of protons will enter into the lumen of the virus through the M2 channel, although this process is thought to be inefficient, because the ion conductance of M2 is generally low at neutral or slightly acidic pH, and there are only a few M2 channels in a single virion. Regardless, protons or other endosomal cues prime the interior of the virus, as indicated by the M1 proteins (blue-shaded) in the viral lumen. This step might occur in early endosomes but it is unknown whether M1 aggregates. As the virus proceeds to more acidic late endosomes, more protons flux into the virus lumen through the acid-gated M2 channel, and, it is thought to lead to disturbance of the M1 layer underneath the viral envelope (38). To see this figure in color, go online.
