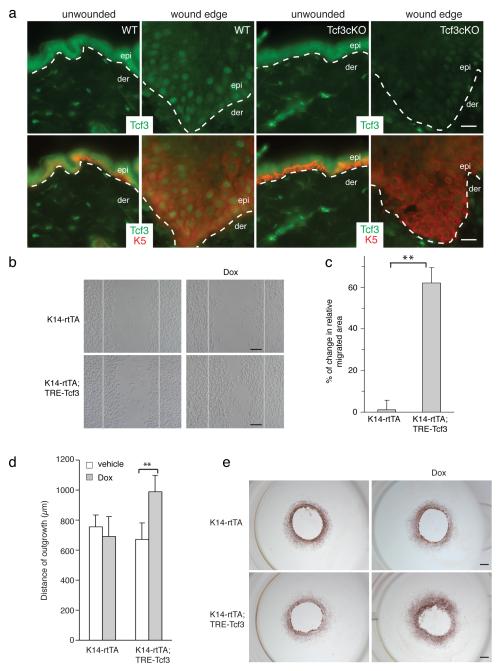
Figure 1. Tcf3 is induced at the wound edge and its overexpression promotes cell migration.
(a) Images of immunofluorescence analysis of wounded and unwounded skins of WT and Tcf3 cKO skins. Full thickness wounds were created on dorsal skins of 10-week old mice and isolated 5 days post wounding. Skins were analyzed by immunofluorescence with antibodies against Tcf3 (green) and keratin 5 (red). Wound-distant skin samples from the same mice were used as unwounded controls. Bar denotes 20μm.
(b) Images of keratinocytes 16hrs after the initiation of migration assay. Primary keratinocytes were isolated from tet-inducible Tcf3 (K14rtTA;TRE-Tcf3) or control (K14-rtTA) mice, grown to confluence, treated with doxycycline (Dox) or vehicle 24hrs prior to being subjected to the migration assay. Cells were treated with Mitomycin C for 2 hours to arrest proliferation, and a scratch was then made in the confluent monolayer using a pipet tip. The size of the scratch was measured at the beginning of the experiment and the area of cell migration was quantified after 16hrs using ImageJ software. Black bar denotes 200μM.
(c) Graph quantifying the area migrated by cells treated with Dox relative to the area migrated by cells treated with vehicle control. For each sample, over 30 non-overlapping fields were measured at each timepoint; and each experiment was repeated twice. Data are the mean ± s.d. **p<0.001 (Student’s t-test).
(d) Graph quantifying the distance of outgrowth (μm) of epithelial cells from skin explants from control (K14-rtTA) or tet-inducible Tcf3 mice (K14-rtTA;TRE-Tcf3). 4-mm dorsal skin punches were cultured for 8 days in the presence or absence of Dox and proliferation was blocked by Mitomycin C treatment on day 3. Explants from a minimum of 4 mice were analyzed for each condition. Data are mean ± s.d. **p<0.01 (Student’s t-test).
(e) Representative images of epithelial outgrowth from explants that were immunostained with keratin 17 antibody and counterstained with hematoxylin after 8 days in culture. Bar denotes 1mm.