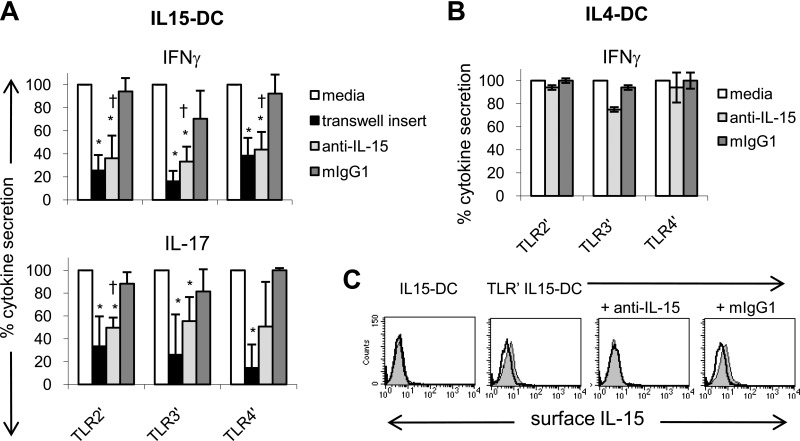
Figure 5. TLR-primed IL15-DCs stimulate IL-17 and IFN-γ secretion by contact- and IL-15-dependent mechanisms.
(A) TLR-primed IL15-DCs were cultured with autologous CD4+ T cells in the presence and absence of 10 μg/ml anti-human IL-15 or mIgG1 for 72 h. In addition, 0.4 μm semipermeable transwell inserts were used to prevent T cells from contacting TLR-primed IL15-DCs. After 3 days, culture fluids were harvested and analyzed for IL-17 and IFN-γ by ELISAs. In each experiment, the cytokine level in cocultures with TLR-primed IL15-DCs (media) was set as 100% and used to calculate the percent cytokine secretion observed with transwell inserts, anti-IL-15, or mIgG1. Data represent the means ± sd of four to five independent experiments. *P < 0.05 between media and transwell or media and anti-IL-15; †P < 0.05 between anti-IL-15 and mIgG1, as determined by the paired two-tailed Student's t test. (B) TLR-primed IL4-DCs were cultured with autologous CD4+ T cells in the presence and absence of 10 μg/ml anti-human IL-15 or mIgG1 for 72 h and culture fluids harvested for IFN-γ ELISA. The percent cytokine secretion observed with and without anti-IL-15 or mIgG1 was calculated as described in A. Data represent the means ± sd of two independent experiments. (C) FACS demonstrating that anti-IL-15 blocks detection of membrane-bound IL-15 on the surface of TLR-primed IL15-DCs, which were incubated with and without antihuman IL-15 or mIgG1 for 1 h and then analyzed for surface expression of IL-15 (filled histograms) by FACS. Unprimed IL15-DCs were included as a negative control. Open histograms depict mIgG1 isotype control staining. One representative experiment is shown.