Abstract
Increased frequency of methicillin-resistant Staphylococcus aureus (MRSA) in hospitalized patients requires rapid and reliable characterization of isolates for control of MRSA spread in hospitals. This study evaluated polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) as a molecular typing technique for MRSA strains on the basis of protein A (spa) and coagulase (coa) gene polymorphisms to verify their ability in assessing the relatedness of isolates. Seventy-five MRSA isolates, from different ICUs of Alexandria University Main Hospital, were characterized using antibiotyping and PCR-RFLP analysis of coa and spa genes. Thirty-two antibiotypes were identified. coa gene PCR generated 3 types and 10 subtypes of band patterns. HaeIII restriction digestion of amplified coa gene products produced 5 major banding patterns and 12 subtypes. spa gene PCR products generated 4 major and 11 minor types, and their HaeII restriction digestion showed 5 major and 12 minor banding patterns. The combined coa and spa RFLP patterns generated 22 combined R types. Typing using coa PCR and PCR-RFLP had the same discriminatory index (DI) value (0.64), which was comparable to that of both spa PCR and PCR-RFLP techniques (0.68). The combined grouping increased the DI value to 0.836. The current study revealed that testing for multiple gene polymorphisms is more useful for local epidemiologic purposes.
1. Introduction
The health risks associated with MRSA infections warrant the implementation of monitoring programs to control its dissemination, given the potential of MRSA to produce invasive infections, particularly in vulnerable patients, and its multiple resistances to antibiotics, which limits the therapeutic options available [1].
Antibiotyping and molecular typing are key functions for epidemiological investigation of hospital-onset S. aureus infection [2].
The antibiogram has been the main typing tool in many hospital outbreaks since the technique is widely available and standardized, and it can be used with all microbial species. Its main disadvantage consists of the variability in resistance expression, which is also susceptible to instability due to horizontal transmission and loss of extrachromosomal genetic elements [1].
Molecular methods for MRSA typing as pulsed field gel electrophoresis (PFGE) have good discriminatory power and more reproducibility but are expensive, not widely available, and time consuming [3]. A method for molecular typing of MRSA that depends on polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) has proven absolute typeability, reproducibility, and good discriminatory power [4].
Variations in the sequence of genes coding for two species-specific proteins, coagulase (coa) and staphylococcal protein A (spa), have been the basis for the most widely used forms of PCR typing for S. aureus, showing a good correlation with PFGE typing [1].
The coagulase gene amplification discriminatory power relies on the heterogeneity of the region containing the 81 bp tandem repeats at the 3′ coding region of the coagulase gene which differs both in the number of tandem repeats and the location of AluI and HaeIII restriction sites among different isolates [5]. Protein A has been coded by spa gene, where the repeated part is located at 3′end and identified as X region; the repetitive part of region X consists of up to 12 units each with a length of 24 nucleotides. This 24-nucleotide region is highly polymorphic with respect to the number and sequence of repeats. Diversity of X region causes protein A variation [6]. In addition to its use as a marker, the number of repeats in the region X of spa has been related to the dissemination potential of MRSA, with higher numbers of repeats associated with higher epidemic capability [1].
The aim of this study was to evaluate PCR-RFLP as a molecular typing technique for MRSA strains on the basis of protein A and coagulase gene polymorphisms and to verify their ability in assessing the relatedness of MRSA isolates.
2. Material and Methods
The material of the study consisted of a total of 100 S. aureus strains isolated from various clinical specimens obtained from patients attending different ICUs in Alexandria Main University Hospital (AMUH) and delivered to the routine laboratory of Microbiology Department at AMUH. All strains were identified as S. aureus according to the standard microbiological techniques [7].
2.1. Antimicrobial Susceptibility Testing and Detection of MRSA
Fully identified S. aureus strains were subjected to antimicrobial susceptibility testing by the Modified Kirby Bauer Disc diffusion method according to the CLSI guidelines (2011) [8], using the following discs: Penicillin (10 U), oxacillin (1 μg), cefoxitin (30 μg), erythromycin (15 μg), gentamicin (10 μg), tetracycline (30 μg), ciprofloxacin (5 μg), vancomycin (30 μg), trimethoprim/sulfamethoxazole (25 μg), amikacin (30 μg), clindamycin (2 μg), chloramphenicol (30 μg), rifampicin (5 μg), and teicoplanin (30 μg) (Oxoid). MRSA strains were further defined as multidrug-resistant (MDR), extensively drug-resistant (XDR), or pandrug-resistant (PDR), guided by the categorization system proposed by the European Society of Clinical Microbiology and Infectious Diseases in 2011 [9]. A standard MSSA strain (ATCC 25923) and a MRSA strain (ATCC 33591) were used as control.
2.2. PCR to Detect mecA Gene [10]
All S. aureus isolates were tested for the presence of the 310 base pair (bp) PCR product of mecA gene, using the following primers: forward (5′-TGG CTA TCG TGT CAC AAT CG-3′) and reverse (5′-CTG GAA CTT GTT GAG CAG AG-3′). MecA positive strain (ATCC 33591) was included as positive control. Amplification reaction was carried out in 25 μL volume, under the following conditions: initial denaturation at 92°C for 5 min, followed by 35 cycles of denaturation at 95°C for 30 seconds, annealing at 56°C for 30 seconds, and extension at 72°C for 30 seconds, followed by final extension at 72°C for 3 min.
2.3. MRSA Typing
(A) PCR of coa Gene [5]. The identified MRSA strains were subjected to PCR for coa gene detection, using the following primers: forward (5′-CGA GAC CAA GAT TCA ACA AG-3′) and reverse (5′-AAA GAA AAC CAC TCA CAT CA-3′), which were designed to amplify the 3′ end hyper variable region containing 81 bp tandem repeats of coa gene. The amplification reaction consisted of an initial denaturation step at 94°C for 5 min, followed by 30 cycles of denaturation at 95°C for 30 sec, annealing at 55°C for 45 sec, extension at 72°C for 2 min, followed by final extension at 72°C for 7 min.
RFLP of coa Gene PCR Products [5]. Depending on the number of 81 bp repeats, a strain analysis of PCR RFLP products was performed with HaeIII restriction enzyme (New England BioLabs, Frankfurt, Germany), where 10 μL of PCR product of coa gene was incubated with 6 U of the enzyme at 37°C for 1 h 45 min in a water bath.
(B) PCR of spa Gene [4]. The identified MRSA strains were subjected to PCR for spa gene detection using the following primers: forward (5′-ATC TGG TGG CGT AAC ACC TG-3′), and reverse (5′-CGC TGC ACC TAA CGC TAA TG-3′) which were designed to amplify the polymorphic X region that contains a variable number of 24 bp tandem repeats of the spa gene coding for protein A. Amplification reaction consisted of an initial denaturation step at 94°C for 4 minutes,followed by 35 cycles of denaturation at 94°C for 1 minute, annealing at 56°C for 1 minute, extension at 72°C for 3 minutes, followed by final extension at 72°C for 5 minutes.
RFLP of spa Gene PCR Products [4, 5]. Five μL of each spa gene amplicon and 10 units of HaeII restriction enzyme (New England BioLabs, Frankfurt, Germany) were incubated at 37°C for 3 hours. The PCR products and restriction digest fragments were detected by electrophoresis in 2% agarose gel.
The interpretation criteria for identifying different strains were a single band difference. Unique PCR-RFLP patterns were assigned a genotype.
2.4. Statistical Analysis: Determination of Numerical Index of Discrimination
The probability that two unrelated isolates sampled from the test population will be placed into different typing groups or clusters was assessed according to the Hunter-Gaston formula [11]. This probability, also called discriminatory index, is calculated using the following equation:
| (1) |
where D = numerical index of discrimination, N = the total number of isolates in the sample population, S = the total number of types obtained, and n j = the number of isolates belonging to this type.
3. Results
A total of 100 S. aureus strains isolated from different clinical specimens and different ICUs were identified according to the standard microbiological techniques. MRSA represented 75% of total S. aureus isolates, while MSSA represented 25% of the strains. Differentiation was based on sensitivity testing using oxacillin and cefoxitin discs, confirmed by the detection of the amplified 310 bp mecA gene using PCR in 75 MRSA strains (Figure 1).
Figure 1.
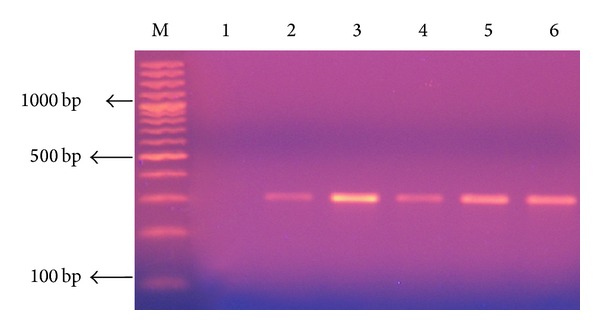
2% Agarose gel electrophoresis analysis of PCR amplification products of mecA gene of 310 bp, extracted from S. aureus. Lane1: negative control (no DNA template); lane 2: positive control (mecA positive strain ATCC 33591); lanes 3–6: methicillin-resistant S. aureus (MRSA); lane M: DNA molecular size marker (100 bp ladder).
3.1. Distribution of MRSA with Respect to Clinical Specimen Type, Admission ICU, Age, and Sex
Among the 75 MRSA isolates, the highest number of isolates was in sputum and tracheal aspirates (46.7%), followed by pus (29.3%), blood cultures (16%), and then urine (6.7%). A single MRSA isolate was isolated from a nasal swab. The highest percentage of MRSA isolates was from ICU 3 (44%), followed by ICU 1 (30.6%), ICU 7 (22.6%), and ICU 6 (2.6%). The age of patients ranged between 15 years and 70 years. The highest percentage of MRSA isolates fell in the age group from >40 to 50 years (26.7%) followed by (21.3%) in age group >30–40 years and (20%) in >20–30 years. Forty-six (61.3%) of MRSA isolates were recovered from male patients.
3.2. Antimicrobial Susceptibility Testing and Antibiotypes of MRSA Isolates
All 75 MRSA isolates were resistant to penicillin, oxacillin, and cefoxitin and all were sensitive to vancomycin and teicoplanin. Resistance to tetracycline, gentamicin, ciprofloxacin, amikacin, erythromycin, chloramphenicol, and clindamycin was high (94.7%, 93.3%, 92%, 89.3%, 73.3%, 69.3%, and 65.3%, resp.), while resistance to rifampin was 36% and that to trimethoprim-sulfamethoxazole was the lowest (18.7%).
Thirty-two antibiotypes were identified. The most common antibiotypes (1 and 2) were found in 16% (12/75) and 10.6% (8/75) of the isolates, respectively. Antibiotype 1 isolates were sensitive to trimethoprim-sulfamethoxazole, intermediately sensitive to rifampin, and resistant to erythromycin, gentamicin, tetracycline, amikacin, chloramphenicol, clindamycin, and ciprofloxacin. Antibiotype 2 isolates were similar to those of antibiotype 1 except that they were sensitive to rifampin. The DI of the antibiogram was (0.94).
3.3. MRSA Genotyping
(1) Coagulase Gene Typing. PCR products of 9 sizes, ranging from 81 to 1215 bp in increments of 81 bp (81, 243, 405, 486, 729, 810, 891, 972, and 1215 bp), were obtained. Pattern electrophoresis analysis generated 3 different types (Co1, Co2, and Co3) and 10 subtypes of band patterns. The majority of MRSA strains (54/75) showed double bands (type Co2), 5/75 showed three bands (type Co3), and the remaining 16/75 strains showed a single PCR band (type Co1) (Table 1 and Figure 2). The most common PCR coa gene product shown was the 405 bp band size product. DI value of coa gene PCR was 0.64.
Table 1.
Combined coa gene typing and HaeIII RFLP patterns of the 75 isolated MRSA strains.
| coa band types (3) | coa gene subtypes (10) | RFLP pattern (12) | Size of PCR product (approximate bp) | Size of HaeIII fragments (approximate bp) | Total isolates number (%) | Isolate serial number |
|---|---|---|---|---|---|---|
|
Co1 (1 band)
16/75 (21.3%) |
Co1a | C1 (3 bands) | 810 | 2 (162), 486 | 3 (4) | 7, 12, 13 |
| Co1b | A1 (uncut) | 891 | 891 | 2 (2.7) | 43, 49 | |
| B (2 bands) | 891 | 324, 567 | 3 (4) | 56, 62, 68 | ||
| C2 (3 bands) | 891 | 2 (162), 567 | 7 (9) | 51–55, 57, 58 | ||
| Co1c | A2 (uncut) | 1215 | 1215 | 1 (1.3) | 46 | |
|
| ||||||
|
Co2 (2 bands)
54/75 (72%) |
Co2a | D1 (4 bands) | 405, 810 | 2 (162), 405, 486 | 44 (58.7) | 1–6, 9–11, 16, 18–20, 22–28, 30–35, 37–42, 45, 47, 50, 60, 61, 65, 66, 71–75 |
| Co2b | D2 (4 bands) | 405, 729 | 81, 162, 405, 486 | 1 (1.3) | 17 | |
| Co2c | D3 (4 bands) | 405, 891 | 2 (162), 405, 567 | 7 (9) | 44, 48, 59, 63, 67, 69, 70 | |
| Co2d | C3 (3 bands) | 81, 810 | 81, 2 (405) | 1 (1.3) | 14 | |
| Co2e | E1 (5 bands) | 405, 972 | 81, 162, 324, 405, 486 | 1 (1.3) | 36 | |
|
| ||||||
|
Co3 (3 bands)
5/75 (6.7%) |
Co3a | E2 (5 bands) | 81, 405, 810 | 81, 2 (162), 405, 486 | 4 (5.3) | 8, 15, 21, 29 |
| Co3b | A3 (uncut) | 243, 486, 1215 | 243, 486, 1215 | 1 (1.3) | 64 | |
Figure 2.
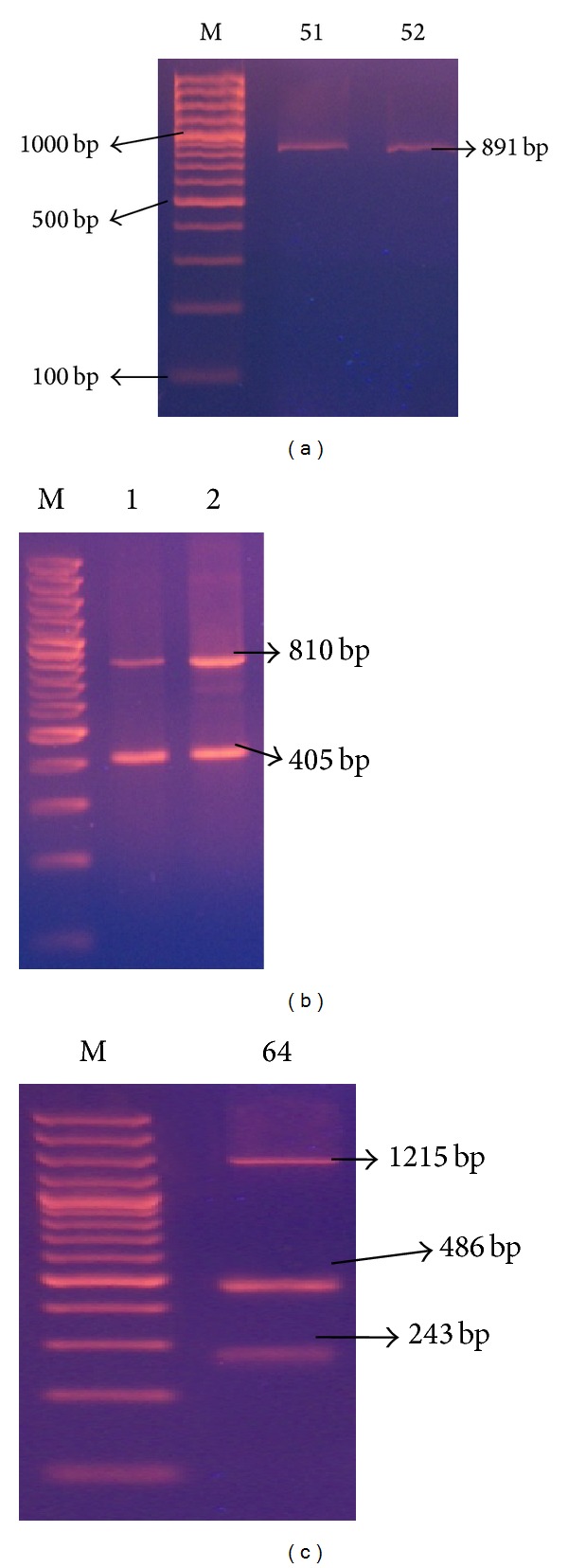
Representative 2% agarose gel electrophoresis of coa gene PCR products where M is DNA molecular size marker (100 bp ladder), (a) isolates 51-52 showing single band, (b) isolates 1-2 showing 2 bands, and (c) isolate 64 showing 3 bands coa gene PCR products.
(2) coa-RFLP Typing Using HaeIII Restriction Enzyme. Restriction digestion was performed on the amplified coagulase PCR products with HaeIII. The bands produced were multiples of 81, divided into 9 band classes. Five distinct RFLP banding patterns (designated as A, B, C, D, and E) and 12 subtypes designated as [A1,2,3/B/C1, 2, 3/D1, 2, 3/E1, 2] were obtained (Table 1 and Figure 3). The majority of strains (44/75 = 58.6%) belonged to RFLP banding pattern D1. PCR products of 4 strains were not digested by HaeIII. Accordingly, this method had 94.6% typeability. DI value of coa PCR-RFLP was 0.64.
Figure 3.
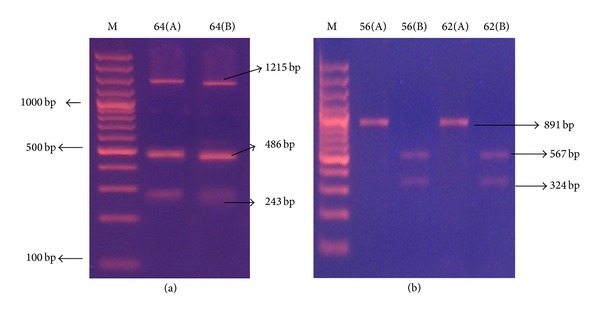
Representative 2% agarose gel electrophoresis of coa gene HaeIII restriction enzyme digestion PCR products, where M is DNA molecular size marker (100 bp ladder), (a) isolate 64 showing 3 bands coa gene PCR products (A) that remained uncut and after (B) cutting with HaeIII restriction enzyme, (b) isolates 56 and 62 showing single band coa gene PCR product (A) and their corresponding 2 bands HaeIII restriction digestion (B).
(3) spa Gene Typing. spa gene PCR products of different sizes could be detected in 71 out of the 75 MRSA isolates. The size of the PCR products ranged from 144 to 1392 bp (144, 192, 240, 360, 408, 600, 1104, 1200, 1296, and 1392), reflecting the number of 24 bp repeat units contained in the spa gene. These PCR products generated 4 major and 11 minor different spa gene types. Sixty-three MRSA strains (84%) showed single PCR band (type S1), 7 (9.3%) had two bands (type S2), and only one (1.3%) strain showed 3 PCR bands (type S3) (Table 2 and Figure 4). The absence of PCR product is considered a separate type and this was shown in 4 strains (S4). DI value of spa gene PCR typing was 0.68.
Table 2.
Combined spa gene typing and HaeII RFLP patterns of the 75 isolated MRSA strains.
| spa band types (4) | spa gene subtypes (11) | RFLP pattern By HaeII (12) | Size of PCR product (approximate bp) | Size of HaeII fragments (approximate bp) | Total isolates number (%) | Isolate serial number |
|---|---|---|---|---|---|---|
|
S1 (1 band)
63/75 (84%) |
S1a | A1 (uncut) | 192 | 192 | 1 (1.3) | 43 |
| S1b | B1 | 1104 | 2 (240), 600 | 3 (4) | 44, 69, 70 | |
| S1c | B2 | 1200 | 2 (240), 696 | 21 (28) | 1–3, 15, 17, 45, 47, 48, 50, 57–59, 63, 66, 67, 71–75 | |
| B3 | 1200 | 2 (192), 792 | 1 (1.3) | 55 | ||
| S1d | B4 | 1392 | 2 (240), 912 | 37 (49.3) | 4–13, 18–35, 37, 38, 40–42, 51–54 | |
|
| ||||||
|
S2 (2 bands)
7/75 (9.3%) |
S2a | A2 (uncut) | 144, 600 | 144, 600 | 1 (1.3) | 64 |
| S2b | C1 | 408, 1296 | 2 (192), 312, 1008 | 1 (1.3) | 14 | |
| S2c | B5 | 240, 1200 | 2 (240), 912 | 3 (4) | 60, 61, 65 | |
| S2d | C2 | 240, 1392 | 2 (240), 504, 792 | 1 (1.3) | 62 | |
| S2e | C3 | 360, 1392 | 100, 240, 504, 912 | 1 (1.3) | 68 | |
|
| ||||||
|
S3 (3 bands)
1/75 (1.3%) |
S3 | D | 360, 1104, 1392 | 150, 2 (240), 792, 456, 912 | 1 (1.3) | 36 |
|
| ||||||
|
S4 (no band)
4/75 (5.3%) |
S4
No band |
E | — | — | 4 (5.3) | 39, 46, 49, 56 |
Figure 4.
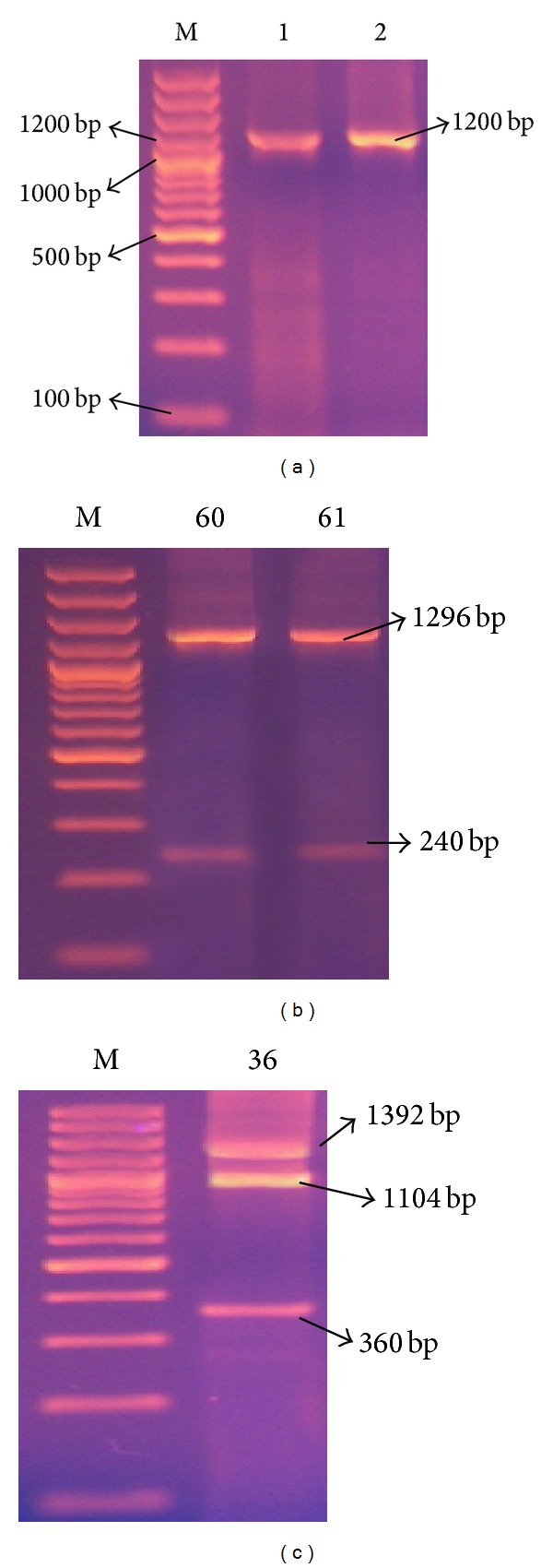
Representative 2% agarose gel electrophoresis of spa gene PCR products where M is DNA molecular size marker (100 bp ladder). (a) Isolates 1 and 2 showing single band PCR products, (b) isolates 60 and 61 showing 2 bands PCR products, and (c) isolate 36 showing 3 bands PCR products.
(4) spa-RFLP Typing and HaeII Restriction Enzyme Digestion. Restriction digestion performed on the amplified spa PCR products with HaeII revealed bands observed to be multiples of 24. Five distinct banding patterns (designated as A, B, C, D, and E) and 12 subtypes designated as [A1, 2/B1, 2, 3, 4, 5/C1, 2, 3/D/E] were produced (Table 2 and Figure 5). Most strains (65/75 = 86.6%) belonged to pattern B especially subtypes B4 (49.3%) and B2 (28%). PCR products of 2 strains were not digested by HaeII and 4 strains had no spa gene band; therefore, this method had 92% typeability. DI value of spa gene RFLP typing method was 0.68.
Figure 5.
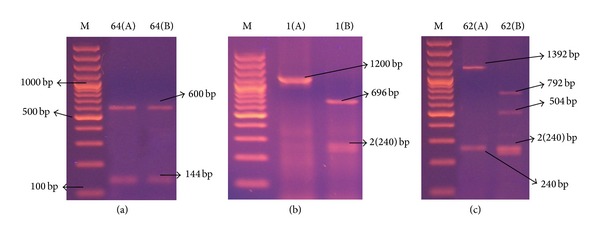
Representative 2% agarose gel electrophoresis of spa gene HaeII restriction enzyme digestion PCR products, where M is DNA molecular size marker (100 bp ladder). (a) isolate 64 showing 2 bands spa gene PCR products (A) that remained uncut after (B) restriction, (b) isolate 1 showing single band spa gene PCR products (A) and the corresponding 3 bands HaeII restriction digestion pattern (B), and (c) isolate 62 showing 2 bands spa gene PCR products (A) and its corresponding 4 bands HaeII restriction digestion products (B).
(5) coa and spa RFLP Combined Analysis (Table 3). The combination of both RFLP patterns was able to identify 22 types (R1–R22), where types R2 and R1 were the most frequent, including 27 and 13 MRSA isolates, respectively. Thirty-three (44%) MRSA strains were from ICU 3, 23 (30.6%) MRSA strains were from ICU 1, 17 (22.6%) from ICU 7, and only 2 (2.6%) from ICU 6. Of the 33 samples from ICU 3, 45.5% of MRSA strains (15/33) were of type R2 and 6/33 (18%) strains belonged to type R1. Concerning the 23 isolates of ICU 1, 34.7% isolates (8/23) were of type R2 and 21.7% (5/23) were of type R1. The remaining isolates were distributed among the different types. The DI value of HaeII and HaeIII combined RFLP classification was 0.836.
Table 3.
Analysis of the discriminatory power of the binary typing method (R) compared with the coa and spa genotyping techniques and antibiogram estimated on the basis of the typing results for the 75 MRSA strains.
| R Type (n) | coa type/RFLP pattern | spa type/RFLP pattern | Isolate origin | Antibiotype |
|---|---|---|---|---|
|
R1 (13)
(5) = ICU 1 (6) = ICU 3 (2) = ICU 7 |
Co2a/D1 | S1c/B2 | Sputum (3) Pus (6) Blood (2) Pus (6) Mini-BAL (1) Urine (1) |
1–3, 7, 19, 20, 30–32 |
|
| ||||
|
R2 (27)
(8) = ICU 1 (15) = ICU 3 (3) = ICU 7 (1) = ICU 6 |
Co2a/D1 | S1d/B4 | Sputum (4) Pus (7) Mini-BAL (9) Blood (3) BAL (2) Urine (2) |
1–7, 9, 11–17, 31 |
|
| ||||
|
R3 (3)
(1) = ICU 1 (1) = ICU 3 (1) = ICU 7 |
Co1a/C1 | S1d/B4 | Wound Pus Mini-BAL |
1, 4 |
|
| ||||
|
R4 (3)
(1) = ICU 1 (1) = ICU 3 (1) = ICU 7 |
Co3a/E2 | S1d/B4 | BAL (2) Sputum (1) |
6, 7, 11 |
|
| ||||
| R5 | Co2d/C4 | S2b/C1 | Bed sore/ICU 7 | 8 |
|
| ||||
| R6 | Co3a/E2 | S1c/B2 | BAL/ICU 3 | 9 |
|
| ||||
| R7 | Co2b/D2 | S1c/B2 | Nasal swab/ICU 3 | 10 |
|
| ||||
| R8 | Co2e/E1 | S3/D | BAL/ICU 7 | 2 |
|
| ||||
| R9 | Co2a/D1 | S4/E | BAL/ICU 6 | 15 |
|
| ||||
| R10 | Co1b/A1 | S1a/A1 | BAL/ICU 1 | 18 |
|
| ||||
|
R11 (3)
(1) = ICU 1 (2) = ICU 3 |
Co2c/D3 | S1b/B1 | Sputum (2) | 17, 19 |
|
| ||||
| R12 | Co1c/A2 | S4/E | Blood/ICU 1 | 21 |
|
| ||||
|
R13 (4)
(2) = ICU 3 (2) = ICU 7 |
Co2c/D3 | S1c/B2 | Mini-BAL Urine BAL Blood |
1, 2, 19, 27 |
|
| ||||
| R14 | Co1b/A1 | S4/E | Wound/ICU 7 | 22 |
|
| ||||
|
R15 (4)
(2) = ICU 1 (1) = ICU 3 (1) = ICU 7 |
Co1b/C2 | S1d/B4 | Blood Urine BAL Sputum |
1, 2, 6, 23 |
|
| ||||
| R16 | Co1b/C2 | S1c/B3 | Pus/ICU 7 | 1 |
|
| ||||
| R17 | Co1b/B | S4/E | Pus/ICU 3 | 1 |
|
| ||||
|
R18 (2)
(1) = ICU 1 (1) = ICU 7 |
Co1b/C2 | S1c/B2 | Wound Mini-BAL |
1, 24 |
|
| ||||
|
R19 (3)
(1) = ICU 1 (1) = ICU 3 (1) = ICU 7 |
Co2a/D1 | S2c/B5 | Wound (2) Blood (1) |
3, 7, 25 3 |
|
| ||||
| R20 | Co1b/B | S2d/C2 | Blood/ICU 3 | 26 |
|
| ||||
| R21 | Co3b/A3 | S2a/A2 | Blood/ICU 1 | 28 |
|
| ||||
| R22 | Co1b/B | S2e/C3 | Blood/ICU 7 | 29 |
DI value of coa PCR-RFLP was not different from DI value of coa gene PCR product (0.64). DI value of spa gene PCR typing was the same as that of spa PCR-RFLP (0.68). DI value of the antibiotyping was the highest (0.948). DI value of HaeII and HaeIII combined classification was 0.836 which is higher than the highest of individual typing (Table 4). So it is desirable that RFLP of both genes should be used for better and reliable discrimination.
Table 4.
Discriminatory indices (DIs) of the binary typing method (R) compared with the coa and spa genotyping techniques and antibiogram for the 75 MRSA strains.
| Method | Types | Size (%) of largest type | Discriminatory index (DI) |
|---|---|---|---|
| coa gene PCR typing | 10 | 44 (58.7%) | 0.64 |
| spa gene PCR | 11 | 37 (49.3%) | 0.68 |
| coa/HaeIII RFLP | 12 | 44 (58.7%) | 0.64 |
| spa/HaeII RFLP | 12 | 37 (49.3%) | 0.68 |
| Binary typing (R) | 22 | 27 (36%) | 0.836 |
| Antibiotyping | 32 | 12 (16%) | 0.948 |
4. Discussion
MRSA is the major cause of nosocomial mortality and morbidity; it is commonly found in the community and hospital environment especially in the ICUs [12].
The present study was conducted on patients attending different ICU wards in AMUH. The backgrounds of the patients (ICU, age, and sex) were randomized to reduce the possibility of contact transmission and various specimens were used for MRSA isolation (respiratory system fluid or secretions, pus, blood, urine, and nasal swabs). Among 100 S. aureus isolated strains, 75 were proven to be MRSA by disc diffusion detection of mecA gene by PCR. These isolates were selected for further genotypic characterization.
Among the 75 MRSA strains, 35 (46.7%) were isolated from respiratory system, 22 (29.3%) from wounds, 12 (16%) from blood samples, 5 (6.7%) from urine samples, and only one sample (1.3%) from a colonized nose. Schmitz et al. [13], in Germany, found that the greatest proportion (17.6%) of their MRSA isolates was from the bronchial/tracheal aspirates.
None of our isolates was resistant to vancomycin or teicoplanin, but all were resistant to penicillin, with variable resistance patterns to the used antimicrobial panel. Using a different panel with few similar antibiotics, Baddour et al. [14] study showed different susceptibility patterns. The difference noted in the susceptibility of the MRSA isolates from different hospitals probably reflects the different patterns of antibiotic usage and thus development of resistance in these hospitals. Antibiotic resistance patterns are influenced by the local environment, selective antibiotic pressure, acquisition, and loss of plasmids carrying resistance genes and various other genetic mechanisms [15]. Sixteen of our isolates could be classified as extensively drug-resistant (XDR). The remaining 59 are multidrug-resistant (MDR) and none was pandrug-resistant (PDR). Other studies documented the association of recovery of MDR-MRSA strains from inpatient clinical samples rather than from outpatients [16, 17].
MRSA typing is an essential component of an effective surveillance system to describe epidemiological trends and infection control strategies. Antibiogram typing has been successfully used for screening of epidemic strains [15]. In the present study, 32 antibiotypes were identified.
Janwithayanuchit et al. [18] from Thailand identified 9 different antibiotypes using a panel of 10 antimicrobial agents. Antibiotypes 1 and 2 represented 44.2% (57/129) and 35.6% (46/129) of their isolates, respectively. Those were nearly similar to our antibiotypes 1 and 2 except in sensitivity to trimethoprim-sulfamethoxazole. They concluded that antimicrobial susceptibility testing is the simplest epidemiologic typing method, the results are easy to interpret, and minimal laboratory skills and equipment are required. Nevertheless, its main disadvantage consists of the variability in resistance expression, which is susceptible to instability due to loss of extra chromosomal genetic elements. However, antibiogram typing cannot be used as the only typing method for MRSA because of its poor discriminatory power [1].
Genotypic techniques to type MRSA must be particularly discriminatory, as MRSA strains probably originate from a single clone or at least a few strain types [13].
The coa gene is the principal criterion for the identification of S. aureus isolates [1]. Its 3′ end contains an 81 bp tandem short sequence repeat (SSR) series, the number of which differs between strains [19]. The coa gene was identified in all of MRSA isolates in this study, indicating 100% typeability. The coa PCR showed 100% sensitivity and specificity in Tiwari et al. (2008) [20] study, confirming the fact that this gene is present in all S. aureus isolates.
In the present study, 9 different amplicons of the coa gene with band sizes ranging from 81 to 1215 bp were found, generating 3 different types and 10 subtypes of coa band patterns. The majority (54/75) of MRSA strains showed double bands, 16 (21.3%) showed single band, and the remaining 5 (6.7%) had three bands. The 405 bp band was the most common and was found in 57/75 of the isolates (76%). The presence of more than one band has been explained by the existence of more than one allelic form of coagulase gene, allowing one strain to produce one or more of these variants [21]. This gene polymorphism might be due to deletion or insertion mutations by which a portion of the 3′ end region of the coa gene is deleted or several nucleotides are inserted and as a consequence change the coa gene size [22].
Himabindu et al. [5], using the same primer, showed that the sizes of coa PCR products were classified into 3 band classes. The majority of isolates belonged to the band class of 812 bp, which was close to our study results, where 69.3% of our isolates belonged to the same band class (810 bp). The difference in coagulase types was found to be subject to geographical variation [23].
According to Lawrence et al. [24], the high discriminative power of coa gene typing in their study was associated with the recovery of a 402 bp PCR product, which was always connected with detection of MRSA in culture. This was in agreement with our finding, where the majority (57 = 76%) of our isolates produced a 405 bp PCR band using the same primers.
In the present study, HaeIII restriction enzyme was chosen for coa gene PCR-RFLP, aiming at a more detailed characterization of the MRSA isolates. It generated 5 RFLP banding patterns (A, B, C, D, and E) and 12 subtypes [A1, 2, 3/B/C1, 2, 3/D1, 2, 3/E1, 2]. Four strains were not digested by the enzyme, indicating that not all the isolates genomic DNA contained HaeIII restriction sites; accordingly, this method had 94.6% typeability.
Lawrence et al. study [24] recovered a 402 bp PCR product which was cut into three fragments of 176 bp, 146 bp, and 81 bp after HaeIII digestion. This was different from our findings where the digestion of the PCR product of band size 405 bp by HaeIII enzyme was unsuccessful, along with the production of other bands (1215 bp, 891, 486, 243, 81) that also remained uncut which might explain the fewer number of genotypes obtained by coa PCR-RFLP in our study.
There is a high degree of heterogeneity in the coa gene of S. aureus making PCR-RFLP of coa gene not suitable as a single typing method [18]. The polymorphisms of the spa and coa genes were not independent, and combination use of them was found by many researchers to be a valid instrument for epidemiological studies [25].
The spa gene is not consistently expressed in all S. aureus isolates [26]. In the present study, spa gene was expressed in 71 isolates (94.6%). X region of spa gene is polymorphic, containing a varying number of 24 bp repeats, whose number and sequence differ among strains [26].
The sizes of the spa PCR products in the current study were classified into 11 classes, creating 4 major and 11 minor different spa gene types according to band number: 63 strains showed single PCR band, 7 had two bands, and only one strain showed 3 PCR bands. The absence of PCR product is considered a separate type and this was shown in 4 strains. This was also shown in Shakeri et al. [6] and Adesida et al. [27] studies, where spa gene was absent in 3.8% and 5% of their S. aureus isolates, respectively.
Similar results were obtained by Schmitz et al. [13] and Montesinos et al. [1], who distinguished, by spa gene PCR, 5 types amongst the 183 and 4 types amongst 124 of their MRSA isolates, respectively.
In addition to its use as a marker, the number of repeats in the region X of spa has been related to the dissemination potential of MRSA, with higher numbers of repeats associated with higher epidemic capability [26]. S. aureus strains with shorter length of protein A cannot adhere to the surface of nasal epithelium and are discharged by breath, sneezing, and coughing [6]. This observation might explain our findings that MRSA strains with longer spa bands (>1200 bp = 1200–1392 bp) were isolated from respiratory secretions (85.6%) more than other samples, reflecting the role of spa protein in adherence to the respiratory epithelium, which protects the isolate from being discharged by breath, sneezing, and coughing.
There is also controversy over the proposed correlation between the number of repeats and the MRSA dissemination potential, with most of the epidemic strains reported as having more than seven repeats. The spread of MRSA strains between hospitals has been recognized in several studies, perhaps as a consequence of the prolonged carrier status and the increased mobility of the population [1]. In the present study, all but one spa gene positive MRSA isolates had more than 7 repeats (>168 bp), and all isolates were disseminated in different ICU wards in the hospital.
HaeII was the restriction enzyme used to digest spa PCR products in the present study, showing 5 distinct spa banding RFLP patterns (A, B, C, and D & E) and 12 minor patterns. The middle sized band (240 bp) remained the same in 67/75 isolates (89.3%).
Closer results were reported by Mehndiratta et al. [28], who reported 5 types of spa gene PCR-RFLP patterns of 1150–1420 bp fragments. However, their smallest fragment that remained the same in all patterns was of size 243 bp.
In Wichelhaus et al. study [4], the discriminatory power of PCR-RFLP of coa and spa genes using HaeII enzyme was found to be sufficiently high and corresponded reasonably well with PFGE. Wilailuckana et al. [29] also reported that typing results of MRSA isolates using combinations of different molecular methods provided a considerably usable tool for discrimination.
In the present study, the combination of both coa and spa genes PCR-RFLP patterns was able to identify 22 combined R types (R1–R22), where types R2 and R1 were the most frequent, including 27 and 13 MRSA isolates, respectively.
A similarly designed study by Mitani et al. [30] reported 8 spa and 6 coa types using HaeII restriction enzyme for coa and spa genes PCR-RFLP. Combination of these 2 techniques revealed 10 R types, where types R1–R4 were relatively frequent.
In the present study, typing using both coa PCR and coa PCR-RFLP had the same DI value (0.64), which is comparable to DI value of typing using both spa PCR and spa PCR-RFLP (0.68). Moreover, the combined grouping of coa PCR-RFLP and spa PCR-RFLP increased the discriminatory index of the typing procedure to 0.836 which is higher when compared to the highest DI of the individual typing technique. So it is desirable that RFLP of both genes should be used for better and reliable discrimination.
The DI value of the antibiotyping in the current study was the highest among all techniques used (0.94). The antibiotic abuse and the lack of antibiotic stewardship in our hospitals could explain the lack of correlation between antibiotypes and genotypes of our MRSA isolates.
This study demonstrates that, although PFGE remains the gold standard for MRSA typing, the typeability of PCR-RFLP products in the genotyping of MRSA strains was an attractive tool for routine epidemiological surveillance and infection control. We therefore recommend this method as a reliable and rapid system in epidemiological investigations similar to this study. Our study revealed that no single gene analysis is efficient in distinguishing strains within a heterogeneous species and testing for multiple gene polymorphisms is more useful for local epidemiologic or outbreak investigation purposes. The polymorphisms of the spa and coa genes are not independently discriminative, and the combined use of both was found to be a valid instrument for epidemiological studies. The variability in genes that are built from repetitive moieties might go along with enhanced speed of evolution. This typing system, therefore, is intended mainly for routine epidemiological surveillance, where isolates obtained within a narrow time frame are subject for typing analysis.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Montesinos I, Salido E, Delgado T, Cuervo M, Sierra A. Epidemiologic genotyping of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis at a University Hospital and comparison with antibiotyping and protein A and coagulase gene polymorphisms. Journal of Clinical Microbiology. 2002;40(6):2119–2125. doi: 10.1128/JCM.40.6.2119-2125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valaperta R, Tejada MR, Frigerio M, et al. Staphylococcus aureus nosocomial infections: the role of a rapid and low-cost characterization for the establishment of a surveillance system. New Microbiologica. 2010;33(3):223–232. [PubMed] [Google Scholar]
- 3.Hookey JV, Richardson JF, Cookson BD. Molecular typing of Staphylococcus aureus based on PCR restriction fragment length polymorphism and DNA sequence analysis of the coagulase gene. Journal of Clinical Microbiology. 1998;36(4):1083–1089. doi: 10.1128/jcm.36.4.1083-1089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wichelhaus TA, Hunfeld K-P, Böddinghaus B, Kraiczy P, Schäfer V, Brade V. Rapid molecular typing of methicillin-resistant Staphylococcus aureus by PCR-RFLP. Infection Control and Hospital Epidemiology. 2001;22(5):294–298. doi: 10.1086/501903. [DOI] [PubMed] [Google Scholar]
- 5.Himabindu M, Muthamilselvan DS, Bishi DK, Verma RS. Molecular analysis of coagulase gene polymorphism in clinical isolates of methicilin resistant Staphylococcus aureus by restriction fragment length polymorphism based genotyping. American Journal of Infectious Diseases. 2009;5(2):163–169. [Google Scholar]
- 6.Shakeri F, Shojai A, Golalipour M, Alang SR, Vaez H, Ghaemi EA. Spa diversity among MRSA and MSSA strains of Staphylococcus aureus in north of Iran. International Journal of Microbiology. 2010;2010:5 pages. doi: 10.1155/2010/351397.351397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird D. Staphylococcus: cluster-forming gram-positive cocci. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. 14th edition. New York, NY, USA: Churchill Livingstone; 1996. pp. 245–258. [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. Performance standards for Antimicrobial Susceptibility Testing; 21th informational supplement. CLSI document M100-S21 Vol 31 No.1. Clinical and Laboratory Standards Institute, Wayne, Pa, USA, 2011.
- 9.Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 10.Vannuffel P, Gigi J, Ezzedine H, et al. Specific detection of methicillin-resistant Staphylococcus species by multiplex PCR. Journal of Clinical Microbiology. 1995;33(11):2864–2867. doi: 10.1128/jcm.33.11.2864-2867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter PR, Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. Journal of Clinical Microbiology. 1988;26(11):2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis SL, Perri MB, Donabedian SM, et al. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. Journal of Clinical Microbiology. 2007;45(6):1705–1711. doi: 10.1128/JCM.02311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz F-J, Steiert M, Tichy H-V, et al. Typing of methicillin-resistant Staphylococcus aureus isolates from Dusseldorf by six genotypic methods. Journal of Medical Microbiology. 1998;47(4):341–351. doi: 10.1099/00222615-47-4-341. [DOI] [PubMed] [Google Scholar]
- 14.Baddour MM, Abuelkheir MM, Fatani AJ. Trends in antibiotic susceptibility patterns and epidemiology of MRSA isolates from several hospitals in Riyadh, Saudi Arabia. Annals of Clinical Microbiology and Antimicrobials. 2006;5, article 30:1–11. doi: 10.1186/1476-0711-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehndiratta PL, Bhalla P. Typing of Methicillin resistant Staphylococcus aureus: a technical review. Indian Journal of Medical Microbiology. 2012;30(1):16–23. doi: 10.4103/0255-0857.93015. [DOI] [PubMed] [Google Scholar]
- 16.Merlino J, Watson J, Rose B, et al. Detection and expression of methicillin/oxacillin resistance in multidrug-resistant and non-multidrug-resistant Staphylococcus aureus in Central Sydney, Australia. Journal of Antimicrobial Chemotherapy. 2002;49(5):793–801. doi: 10.1093/jac/dkf021. [DOI] [PubMed] [Google Scholar]
- 17.Awadalla HI, Khalil IA, Bassim HH, Ahmed MN, Wahba LM. Molecular typing of methicilin-resistant Staphylococcus aureus isolates at Ain Shams University Hospital, Egypt. African Journal of Microbiology Research. 2010;4(15):1639–1646. [Google Scholar]
- 18.Janwithayanuchit I, Ngam-ululert S, Paungmoung P, Rangsipanuratn W. Epidemiologic study of methicillin-resistant Staphylococcus aureus by coagulase gene polymorphism. ScienceAsia. 2006;32(2):127–132. [Google Scholar]
- 19.Shopsin B, Gomez M, Waddington M, Riehman M, Kreiswirth BN. Use of coagulase gene (coa) repeat region nucleotide sequences for typing of methicillin-resistant Staphylococcus aureus strains. Journal of Clinical Microbiology. 2000;38(9):3453–3456. doi: 10.1128/jcm.38.9.3453-3456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiwari HK, Sapkota D, Gaur A, Mathuria JP, Singh A, Sen MR. Molecular typing of clinical Staphylococcus aureus isolates from Northern India using coagulase gene PCR-RFLP. Southeast Asian Journal of Tropical Medicine and Public Health. 2008;39(3):467–473. [PubMed] [Google Scholar]
- 21.Goh S-H, Byrne SK, Zhang JL, Chow AW. Molecular typing of Staphylococcus aureus on the basis of coagulase gene polymorphisms. Journal of Clinical Microbiology. 1992;30(7):1642–1645. doi: 10.1128/jcm.30.7.1642-1645.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saei HD, Ahmadi M, Mardani K, Batavani RA. Molecular typing of Staphylococcus aureus isolated from bovine mastitis based on polymorphism of the coagulase gene in the north west of Iran. Veterinary Microbiology. 2009;137(1-2):202–206. doi: 10.1016/j.vetmic.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Lange C, Cardoso M, Senczek D, Schwarz S. Molecular subtyping of Staphylococcus aureus isolates from cases of bovine mastitis in Brazil. Veterinary Microbiology. 1999;67(2):127–141. doi: 10.1016/s0378-1135(99)00031-0. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence C, Cosseron M, Mimoz O, et al. Use of coagulase gene typing method for detection of carrier of methicillin resistant Staphylococcus aureus . Journal of Antimicrobial Chemotherapy. 1996;37:687–696. doi: 10.1093/jac/37.4.687. [DOI] [PubMed] [Google Scholar]
- 25.Saei HD, Ahmadi M. Discrimination of Staphylococcus aureus isolates on the basis of gene coding protein A using PCR-restriction enzyme analysis. Comparative Clinical Pathology. 2011;21(5):645–652. [Google Scholar]
- 26.Frenay HME, Theelen JPG, Schouls LM, et al. Discrimination of epidemic and nonepidemic methicillin-resistant Staphylococcus aureus strains on the basis of protein A gene polymorphism. Journal of Clinical Microbiology. 1994;32(3):846–847. doi: 10.1128/jcm.32.3.846-847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adesida SA, Likhoshvay Y, Eisner W, et al. Repeats in the 3′ region of the protein A gene is unique in a strain of Staphylococcus aureus recovered from wound infections in Lagos, Nigeria. African Journal of Biotechnology. 2006;5(20):1858–1863. [Google Scholar]
- 28.Mehndiratta P, Bhalla P, Ahmed A, Sharma Y. Molecular typing of methicillin-resistant Staphylococcus aureus strains by PCR-RFLP of SPA gene: a reference laboratory perspective. Indian Journal of Medical Microbiology. 2009;27(2):116–122. doi: 10.4103/0255-0857.45363. [DOI] [PubMed] [Google Scholar]
- 29.Wilailuckana C, Tribuddharat C, Tiensasitorn C, et al. Discriminatory powers of molecular typing techniques for methicillin-resistant Staphylococcus aureus in a University Hospital, Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 2006;37(2):327–334. [PubMed] [Google Scholar]
- 30.Mitani N, Koizumi A, Sano R, et al. Molecular typing on methicillin-resistant Staphylococcus aureus by PCR-RFLP and its usefulness in an epidemiological study of an outbreak. Japanese Journal of Infectious Diseases. 2005;58(4):250–252. [PubMed] [Google Scholar]


