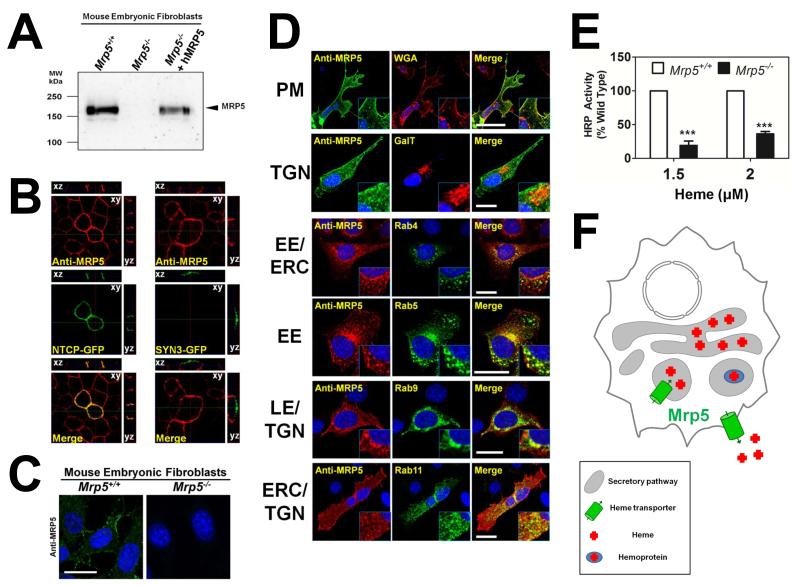
Figure 6. MRP5 localizes to the secretory pathway and alters heme levels in this compartment.
(A) Immunoblot analysis of Mrp5 expression in MEFs generated from Mrp5+/+ and Mrp5−/− FVB mice. Cell lysates were resolved on SDS/PAGE and blotted to nitrocellulose membranes for probing with a monoclonal anti-MRP5 antibody. (B) MDCKII cells stably expressing human MRP5 were transfected with the basolateral marker NTCP-GFP or the apical marker Syntaxin3-GFP and grown to confluency on transwell filters. Polarization of the monolayer was determined after measuring a transient spike in trans-epithelial electrical resistance, which remained above baseline level. Cells were fixed and probed with monoclonal anti-MRP5, followed by Alexa 568-conjugated secondary antibody and imaged using confocal microscopy. A single confocal section (xy) is depicted along with composite stacks in side views (yz, xz). (C) Immunohistochemistry of endogenous MRP5 in Mrp5+/+ and Mrp5−/− mouse MEFs. MRP5 staining was performed as in Figure 6B, using an Alexa 488-conjugated secondary antibody. Scale bar, 20 μM. (D) Immunolocalization of human MRP5 overexpressed in MEFs by confocal microscopy. WGA is used as a plasma membrane (PM) marker, RFP-GalT as a trans-Golgi (TGN) marker, Rab4YFP marks early endosomes (EE) and the endocytic recycling compartment (ERC), Rab5YFP marks EEs, Rab9YFP marks late endosomes (LE) and the TGN, Rab11YFP marks the ERC and TGN. Scale bar, 20 μM. (E) Heme-dependent horseradish peroxidase activity in Mrp5+/+ or Mrp5−/− MEFs. Cells were transfected with GolgiHRP and then grown for 24 hr in heme-depleted media plus succinyl acetone (HD+SA) for complete heme depletion. Indicated amounts of heme were added back and cells were incubated for a further 24 h. Cell lysates were harvested and analyzed for peroxidase activity, which was normalized to peroxidase activity from samples not expressing GolgiHRP and then to the protein concentration of each sample. ***P<0.001 for knockout MEFs when compared to wildtype MEFs under identical conditions, n=3 (two-way ANOVA, Bonferonni post-test). (F) Proposed model for heme transport by MRP-5: in this composite model, based on results from genetic, biochemical, and localization studies in worm and mammalian systems, MRP-5 can localize to the plasma membrane for heme export as well as to the secretory pathway for heme delivery to luminal hemoproteins.