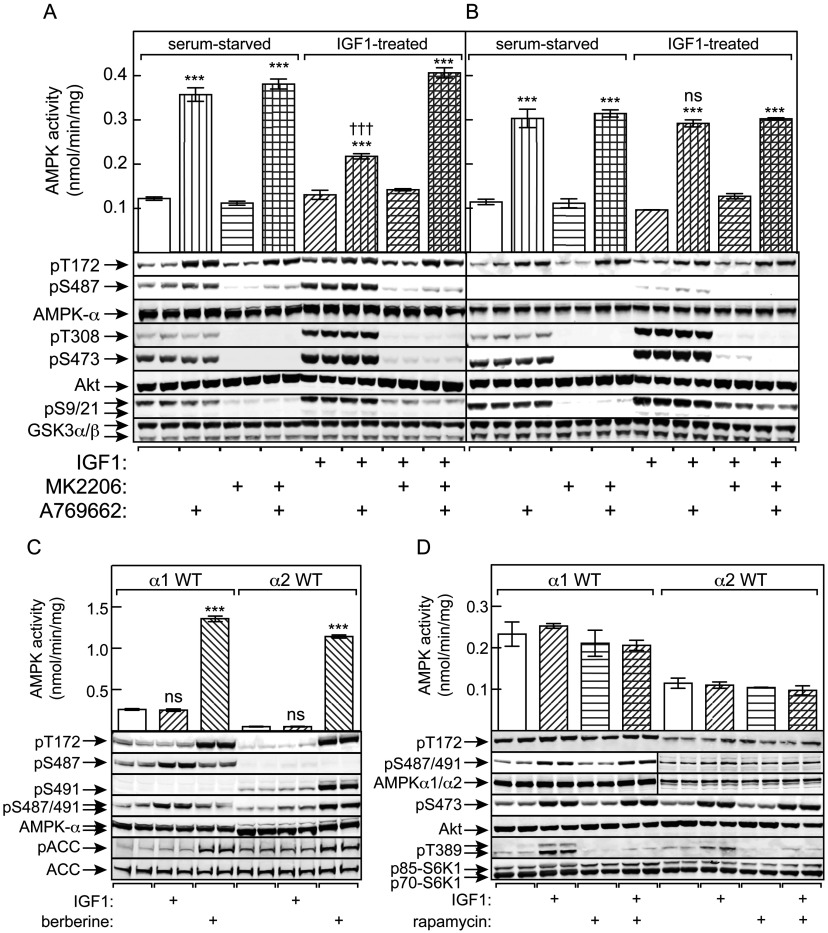
Figure 4. Phosphorylation of Ser487 on AMPK-α1 by Akt in HEK-293 cells inhibits subsequent phosphorylation of Thr172 and AMPK activation, Ser491 on AMPK-α2 is modified by autophosphorylation, and Ser487 phosphorylation is rapamycin-insensitive.
HEK-293 cells stably expressing WT AMPK (A) or an S487A mutant (B) were serum-starved overnight and then incubated with IGF-1 in the presence or absence of MK2206 as described in the Experimental section. The cells were then treated with or without A769662 (300 μM for 40 min) and lysates prepared for immunoprecipitate kinase assay and Western blots. Blots are samples from separate dishes (n=2), whereas activity data are means±S.E.M. (n=4); ***P<0.001 compared with relevant control without A769662; †††P<0.001; ns, not significant, for IGF-1-treated against relevant serum-starved control. (C) Cells expressing WT AMPK-α1 or AMPK-α2 were treated with IGF-1 (30 ng/ml) or berberine (300 μM) and lysates analysed by immunoprecipitate kinase assays and Western blotting (two separate dishes). Activity data are means±S.E.M. (n=4); ***P<0.001; ns, not significant, compared with control without IGF-1 or berberine. (D) Cells expressing WT AMPK-α1 or AMPK-α2 were treated with IGF-1 in the absence or presence of rapamycin (100 μM) and lysates analysed by immunoprecipitate kinase assays and Western blotting. Activity data are means±S.E.M. (n=2); duplicate blots were from separate dishes.