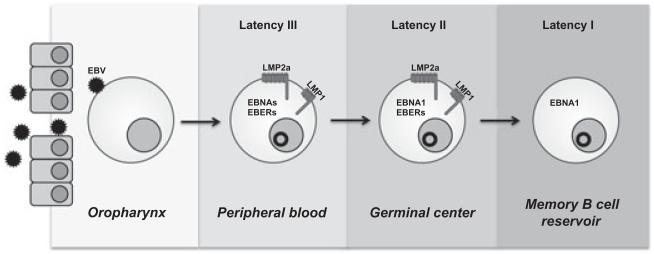
Fig. 1.
Viral gene expression during the life cycle of EBV. EBV preferentially establishes an infection in naïve, tonsillar B cells. While a minority of infected cells remains permissive for the production of infectious viral particles, a latent infection is established in the majority of infected cells. Infected cells displaying this form of latency (latency III) express the EBV nuclear antigens (EBNAs) 1, 2, 3A, 3B, 3C, LP, LMP1 and LMP2a, and EBV small RNAs (EBERs 1, 2). Expression of a type III latency has been proposed to promote activation of the naïve B cell to become a proliferating lymphoblast, similar to B-cell activation by antigen. Infected cells then transition through a latency II, which is characterized by the loss of EBNA 2, 3A, 3B, 3C, and LP expression. During latency II, LMP1 (the CD40 homolog of EBV) and LMP2a (the BCR homolog of EBV) have been proposed to provide signals independent of the antigen-driven interactions with TH cells or FDCs that are required for B-cell differentiation through the GC and into the memory B-cell compartment. Finally, EBV persists for the lifetime of the host in the peripheral memory B-cell reservoir by switching to a type I latency. In this program, latently infected cells escape immunosurveillance by EBV-specific cytotoxic T lymphocytes by not expressing any latent genes. To ensure passage of the viral episome during cell division, the poorly immunogenic EBNA1 is periodically expressed in latently infected memory B cells.