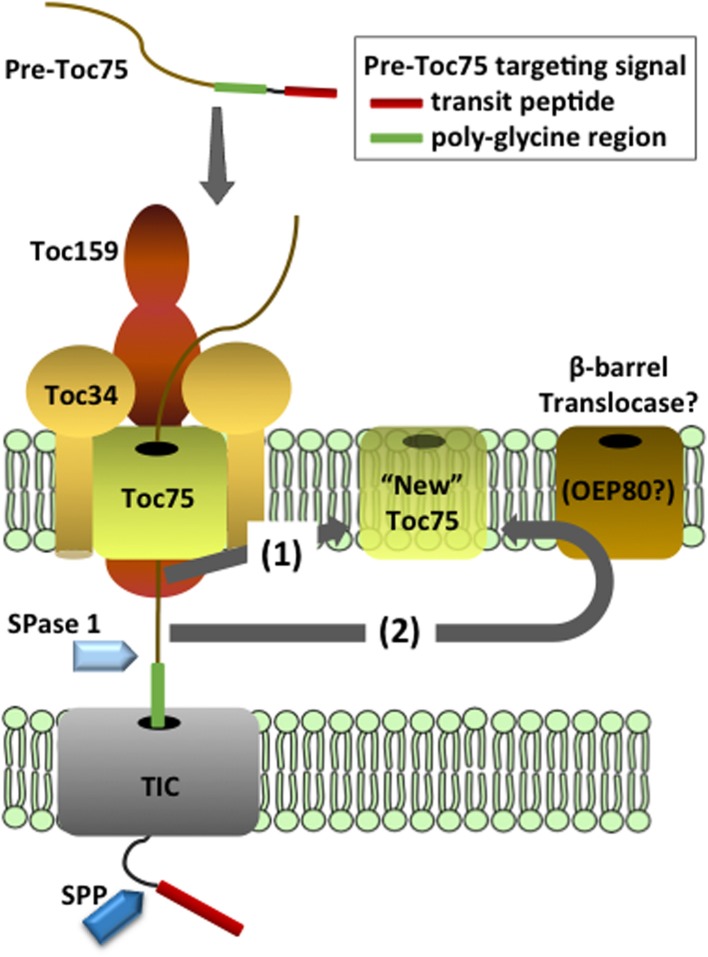
Figure 2.
Models for the targeting and insertion of Toc75. Pre-Toc75 is synthesized with a bipartite targeting signal containing an N-terminal transit peptide followed in tandem by a poly-glycine region. The transit peptide targets pre-Toc75 to the TOC complex and initiates membrane translocation through the TOC and TIC channels. The stromal processing peptidase cleaves the transit peptide to generate an intermediate form (iToc75), and the poly-glycine region halts complete translocation of iToc75 in the intermembrane space. Two hypotheses have been proposed for the insertion of iToc75 into the outer membrane. Pathway (1) proposes that insertion is mediated directly by the TOC complex. Pathway (2) proposes that iToc75 is engaged by a β–barrel translocase of unknown composition, which catalyzes membrane insertion from the intermembrane space. OEP80 has been proposed as a core constituent of the β–barrel translocase. iToc75 is processed by a type I signal peptidase (SPase 1) to yield mature Toc75 during or shortly after insertion in the outer membrane.