Figure 1.
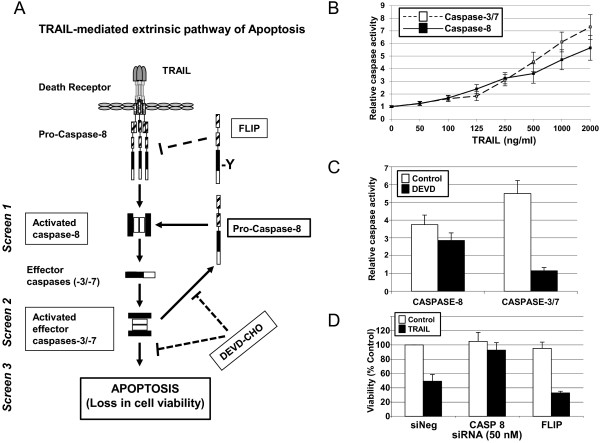
The development of siRNA-based RNAi screens for the identification of regulators of TRAIL-induced apoptosis in the MB231 breast cancer cell line. (A) A diagrammatic representation of the extrinsic TRAIL-induced apoptotic pathway. RNAi screens were developed assaying caspase-8 activation (Screen 1), caspase-3/7 activation (Screen 2), and cell viability (Screen 3) in the absence and presence of TRAIL. Synthetic siRNAs corresponding to CASP8 and FLIP were used as positive and negative regulator controls of the TRAIL pathway, respectively. The caspase-3/7 DEVD-CHO inhibitor is shown on the diagram. (B) Caspase-3/7 and caspase-8 activity was measured by using caspase-3/7 and caspase-8-Glo assays. MB231 cells were treated with increasing concentrations of TRAIL (as indicated) or RPMI medium for 1 hour, after which caspase activity was measured. Fold-increase in caspase activity is plotted relative to the untreated cells. Data are shown as the mean and standard error of three experiments. (C) Caspase-3/7 and caspase-8 activity were measured after pretreatment with or without 0.03 μM DEVD-CHO for 1 hour and then treatment with 1,000 ng/ml TRAIL for 1 hour. Inhibition with DEVD-CHO blocked caspase-3/7 activity significantly compared with caspase-8 activity. Data are shown as the mean and standard error of three experiments. (D) Viability of MB231 cells was measured by an MTS assay 48 hours after the transfection of the negative-control siRNA (siNeg) or siRNAs corresponding to CASP8 and FLIP, respectively, either in the absence or presence of 1,000 ng/ml TRAIL for 17 hours. Data are shown as the mean and standard error of three experiments. (E) Western-blot analysis of cell lysates for CASP8 and FLIP expression, 48 hours after the transfection of the negative control siRNA (siNeg) or siRNAs corresponding to CASP8 and FLIP, respectively.
