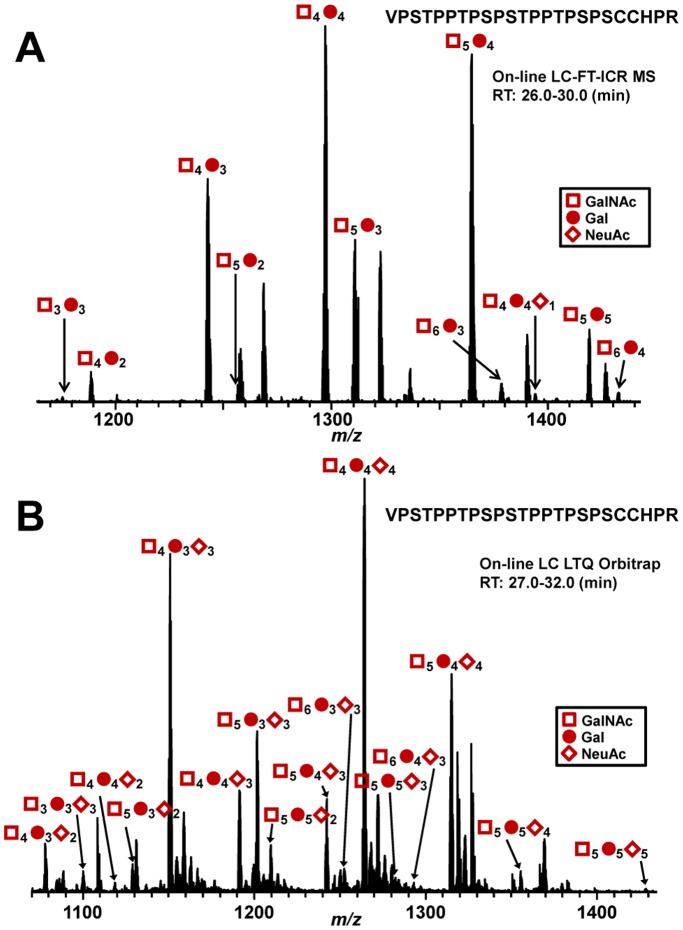
Figure 3. MS analysis of sialylation by ST3Gal1 of IgA1 myeloma protein that is naturally sialic-acid-deficient.
(A) HR O-glycan profile of IgA1 (Ale) myeloma protein. The number of O-glycans was assigned based on the masses of the amino-acid sequence, GalNAc (empty squares), Gal (full circles), and NeuAc (full diamonds). The O-glycans of the protein are minimally sialylated. All HR O-glycoforms are ionized as triply charged ions. (B) HR O-glycan profile of IgA1 (Ale) myeloma protein after over-night sialylation reaction with ST3Gal1. The enzyme added NeuAc residues to the O-glycans of Ale myeloma protein. All HR O-glycoforms are ionized as quadruply charged ions.