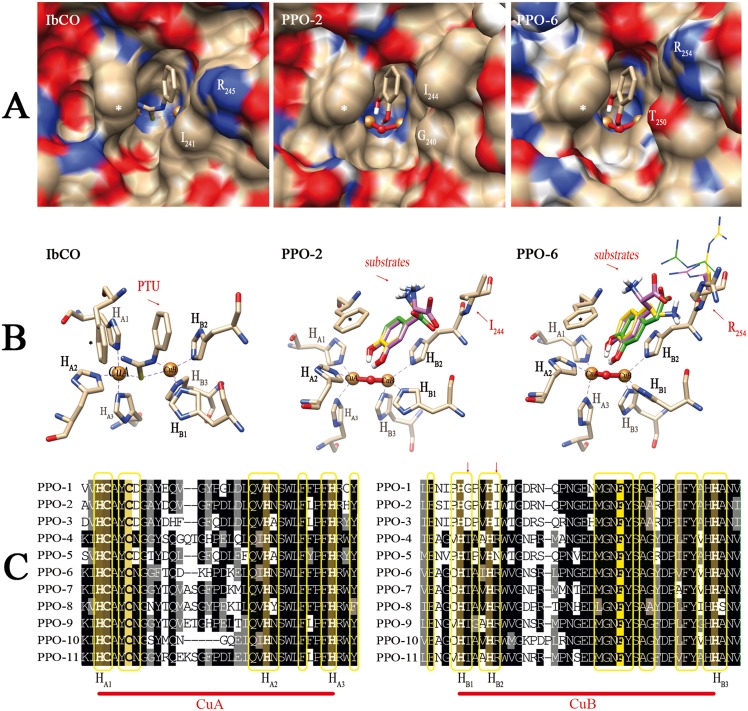
Figure 6. Molecular modeling and docking studies of dandelion PPOs.
PPO-2 and PPO-6 are shown here as representatives of each phylogenetic group. Surfaces and stick models are colored according to atom type (blue, nitrogen; red, oxygen; gray, carbon; white, hydrogen) and the following special designations: * = gate residue (phenylalanine); gold spheres = copper atoms; red sphere = bound oxygen in met-form. (A) Surface contour images of the catalytic pocket, showing the crystallographic structure of IbCO (PDB 1BUG,A) and homology models of PPO-2 (group 1) and PPO-6 (group 2). The substrate analog phenylthiourea (PTU, stick representation) occupies the substrate binding site in the hydrophobic cavity. For comparison, the binding of CAT (stick representation) as the simplest substrate is shown in the active site for the modeled dandelion PPOs. Residues HB1+1 and HB2+1 are labeled at the entrance of the catalytic pocket. (B) Predicted interactions of the substrate in the active site resulting from docking analysis. The position and interaction of IbCO with PTU is shown as a reference. PPO-2 and PPO-6 are shown binding to Dopac (green), DA (yellow) and L-Dopa (purple); only polar hydrogen atoms are drawn for the substrates. For PPO-6, different R254 side chain rotamers resulted from binding of the different substrates. The rotamers are drawn as thin stick and are colored green, yellow or purple according to the corresponding substrate. (C) Sequence alignment of the copper-binding sites (CuA and CuB) of all eleven dandelion PPOs. Identical and similar residues are shaded in black and gray, respectively. The Cu-binding histidine residues (HA1–A3, HB1–B3) are labeled, and arrows indicate the HB1+1 and HB2+1 positions. The gate residue is shaded in yellow. Residues located within 8Å of the copper centers in 3D space are boxed in yellow.