Abstract
Background
Major depressive disorder (MDD) is associated with functional abnormalities in fronto-meso-limbic networks contributing to decision-making, affective and reward processing impairments. Such functional disturbances may underlie a tendency for enhanced altruism driven by empathy-based guilt observed in some patients. However, despite the relevance of altruistic decisions to understanding vulnerability, as well as everyday psychosocial functioning, in MDD, their functional neuroanatomy is unknown.
Methods
Using a charitable donations experiment with fMRI, we compared 14 medication-free participants with fully remitted MDD and 15 demographically-matched control participants without MDD.
Results
Compared with the control group, the remitted MDD group exhibited enhanced BOLD response in a septal/subgenual cingulate cortex (sgACC) region for charitable donation relative to receiving simple rewards and higher striatum activation for both charitable donation and simple reward relative to a low level baseline. The groups did not differ in demographics, frequency of donations or response times, demonstrating only a difference in neural architecture.
Conclusions
We showed that altruistic decisions probe residual sgACC hypersensitivity in MDD even after symptoms are fully remitted. The sgACC has previously been shown to be associated with guilt which promotes altruistic decisions. In contrast, the striatum showed common activation to both simple and altruistic rewards and could be involved in the so-called “warm glow” of donation. Enhanced neural response in the depression group, in areas previously linked to altruistic decisions, supports the hypothesis of a possible association between hyper-altruism and depression vulnerability, as shown by recent epidemiological studies.
Keywords: Charitable donation, Major depression, Reward processing, Subgenual anterior cingulate, Striatum
Highlights
-
•
Patients show enhanced activation in the sgACC while making altruistic decisions.
-
•
Patients show elevated STR response to equitable decisions.
-
•
These abnormal neural responses may be associated with depression vulnerability.
1. Introduction
Charitable donation behaviour is a unique form of human altruism which challenges kin selection theories of interpersonal helping behaviours (Hamilton, 1963; Foster et al., 2006). The “warm glow utility model” posits that people engage in helping behaviours because they are socially rewarding and pleasurable (Andreoni, 1990). The avoidance of anticipated guilt may be another important motivator of altruistic behaviour (Eisenberg, 2000; Tangney et al., 2007). Major depressive disorder (MDD) is associated with elevated levels of self-blaming moral emotions such as shame and guilt (see reviews: Kim et al., 2011; Pulcu et al., 2013); particularly survivor guilt (O'Connor et al., 2000) which persists into remission (Green et al., 2013). It has been suggested that empathy-based guilt is associated with hyper-altruism in MDD (O'Connor et al., 2012). Epidemiological studies support this view and suggest that hyper-altruistic tendencies (e.g. making donations exceeding $10/month) constitute a vulnerability factor for the first onset of MDD (Fujiwara, 2009). This suggests that charitable donation, perhaps acting as an index of empathy-based guilt, may represent a trait marker for MDD. A potential neuronal basis of this effect has not been investigated.
Previous neuroimaging studies of charitable donation behaviour in healthy participants suggested selectively enhanced response of septal and subgenual cingulate (sgACC) regions during decisions to make donations relative to decisions to accept simple monetary rewards (Moll et al., 2006). The septal part of the nucleus accumbens (Harbaugh et al., 2007; Hsu et al., 2008) and an anterior ventral area of the ventromedial frontal cortex (Hare et al., 2010) have also been associated with donation decisions. The sgACC was also found to be more active in people with higher empathic concern while they made decisions to sacrifice money to help others (Feldman Hall et al., 2012). The authors of these studies argue that the sgACC may play a critical role in processing prosocial and affiliative emotions as well as moral decisions. The sgACC has reproducibly been found to selectively respond while people experience guilt, which may relate to its involvement in donation decisions (Zahn et al., 2009a, 2009c; Green et al., 2012a, Basile et al., 2011a; Morey et al., 2012).
In contrast to the selective involvement of septal and subgenual cingulate regions in a previous study of altruistic donation decisions relative to selfish rewards, activation in the lateral striatum was common to both altruistic and simple monetary reward decisions (Moll et al., 2006), consistent with the commonly reported role of striatal structures in processing financial (and other) rewards (Diekhof et al., 2012). Striatal response was also observed more strongly when people made donations in the presence of a social audience, therefore receiving additional social rewards such as recognition and appraisal (Izuma et al., 2010). These studies suggest that septal/subgenual regions distinguish altruistic decisions from those that increase individuals' own financial resources, potentially reflecting guilt or other prosocial processes, whereas striatal regions respond both to outcome of altruistic decisions and to simple receipt of money, potentially reflecting reward-related response.
A well-established clinical literature suggests that MDD is associated with both structural (Drevets et al., 1997; Drevets et al., 1998; Botteron et al., 2002; Drevets and Savitz, 2008) and functional impairments of the sgACC during the symptomatic phase (Mayberg et al., 1997; Mayberg et al., 2000; Mayberg et al., 2005; Siegle et al., 2006; Lehmbeck et al., 2008), with abnormalities in functional connectivity (Greicius et al., 2007). Connectivity abnormalities extend well into remission while patients are processing guilt (Green et al., 2012b). Studies also suggest functional impairments of reward processing systems, including the striatum, even when symptoms fully remit (Tremblay et al., 2005; Schlaepfer et al., 2008; Knutson et al., 2008; Eshel and Roiser, 2010). These previous functional neuroimaging studies have shown enhanced sgACC, but blunted striatal response in MDD across different reward, affective and social processing paradigms, even in remission. However, best to our knowledge there is no study which has investigated brain imaging correlates of social reward processing impairments in remitted or current MDD. Based on the evidence reviewed above, we suggest that decisions to make charitable donations may be an experimental probe for understanding functional impairments related to social decision-making, associated with abnormality of fronto-meso-limbic networks.
Here, we used functional magnetic resonance imaging (fMRI) with an experimental charitable donations paradigm to investigate the neural bases of altruistic decisions in MDD. Trait abnormalities in donation behaviour have been previously suggested by an epidemiological study (Fujiwara, 2009). In order to investigate the neural basis of donation behaviour associated with depression vulnerability, we recruited unmedicated patients with MDD fully remitted from symptoms (rMDD) and a group of matched controls with no personal or family history of MDD. A growing number of studies suggest that using functional neuroimaging in rMDD is a valid approach to investigating biological trait markers for future major depressive episodes (Bhagwagar and Cowen, 2008; Dichter et al., 2012; Elliott et al., 2012; Nixon et al., 2014; Schiller et al., 2013; Pulcu et al., 2014). Studying remitted MDD has additional advantages such as mitigating the effects of current mood state and antidepressant medications (Dichter et al., 2012; Schiller et al., 2013). Here, we investigated the following hypotheses, based on previous literature reviewed above: compared with controls, people with rMDD would exhibit 1) enhanced sgACC response to donation decisions relative to simple monetary rewards and 2) reduced responses to rewards in striatal regions (e.g. septal, nucleus accumbens, caudate, globus pallidus).
2. Material and methods
2.1. Participants
We obtained ethical approval from the North West/Manchester South NHS Research Ethics Committee. Participants were recruited using online and print advertisements. Initial suitability was assessed with a phone pre-screening interview and an online survey. Written informed consent was obtained from all participants.
2.1.1. Inclusion/exclusion of participants
Patients with rMDD fulfilled the criteria for a past major depressive episode in full remission according to DSM-IV-TR (American Psychiatric Association, 2000). The clinical interviews were conducted by trained researchers (see below). We excluded people with current MDD, current or history of substance use disorders, psychotic disorders, bipolar depression, any Axis-I anxiety disorders diagnosed prior to the initial major depressive episode or any history of neurological disorders. We also excluded patients using psychotropic medication. The healthy control group additionally had no current or past Axis-I disorders and had no first-degree relatives with a history of Axis-I disorders. All participants had normal or corrected-to-normal vision.
In total, 15 healthy control participants and 14 individuals with rMDD (see Supplementary materials Table 1 for further clinical information) were included in the final analysis. One patient with rMDD and one healthy subject were excluded because of an insufficient number of acceptances in the simple financial reward condition (see below for the details of the fMRI paradigm).
2.2. Clinical interview procedure
Participants were invited for a clinical interview in which trained researchers (EJT or PDT) conducted the Mood Disorders Module A and the psychotic screening of the Structured Clinical Interview for DSM-IV-TR (SCID) (First et al., 2002). The MINI screening (Mini International Neuropsychiatric Interview; Sheehan et al., 1998) was conducted with all participants and relevant Structured Clinical Interview for DSM-IV-TR (SCID) modules were used in order to make a full assessment. The Montgomery Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) and the Global Assessment of Functioning (GAF) scale (Axis V, DSM-IV) were used to assess current symptoms and social functioning.
2.3. fMRI paradigm
The charitable donations task was adapted from Moll et al. (2006) and the choice of charities was based on the findings of a previous pilot study, which investigated people's perceptions and preferences about charitable organisations in England and Wales. Mission statements of 95 charities were obtained from The Charity Commission for England and Wales (http://www.charity-commission.gov.uk/) for the pilot study. The 36 charitable organisations with the most positive mission statement ratings in the pilot were selected for the functional neuroimaging task. Unlike the Moll et al. (2006) study, our imaging paradigm did not contain any charities probing costly/non-costly opposition behaviour. This decision was based on the findings of the pilot study in which we only identified 11 charities (predominantly focusing on controversial religious themes) with mildly negative mission statement ratings (a detailed analysis of the pilot study is available from the authors upon request). Our pilot findings may reflect a significant cultural difference in charities between the US and the UK. US charities include political organisations and lobbying groups, for example charities promoting gun control or abortion, which elicit strong opposition in some people and strong support in others (Wright, 2002). Registered UK charities are generally less politicised and, while people are more or less likely to support these charities, there are very few organisations which people would actively oppose (and still fewer which people would pay to oppose).
Before the fMRI experiment, participants were given a document containing the full name and the mission statement of 36 charities and the payoff conditions were explained to them (see Table 1 for payoffs and their comparison with Moll et al. (2006)).
Table 1.
The payoffs for the participant and the charity across different conditions and comparisons between Moll et al. (2006).
| Participant | Charity | Total number of conditions (out of 144) | Participanta | Charitya | Total number of conditionsa | |
|---|---|---|---|---|---|---|
| Funds | £20 | N/A | N/A | $128 | N/A | N/A |
| Costly | -30p | +£1 | 36 | -$2 | +$5 | 32 |
| Non-costly | -0p | +£1 | 36 | -$0 | +$5 | 32 |
| Reinforcing | +10p | +£1 | 24 | N/A | N/A | N/A |
| Reward | +10p | +£0 | 24 | +$2 | +$0 | 32 |
| Neutral | -0p | +£0 | 24 | N/A | N/A | N/A |
| Null-fixation | + | + | 144 | + | + | 48 |
| Penalty | -20p | N/A | N/A | -$1 | N/A | N/A |
The financial magnitude of proposals in the current study is written in bold (i.e. left side of the table). aDetails and the frequency of the conditions in the Moll et al. (2006) study is presented on the right side of the table. Moll et al. (2006) also contained costly and non-costly opposition proposals which were not included in the present study due to differences in charitable giving between the US and the UK. At the time of writing this manuscript, $2 converted to £1.20. The main difference between the studies is the relative magnitudes of financial components of the conditions; for Moll et al. (2006): penalty < reward = costly donation < charity amount; whereas in the present study: reward < penalty < costly donation < charity amount. Greater financial magnitude for penalty over reward is preferred in the present design in order to reduce the number of trials with no responses. The (+) denotes the pattern of fixation. N/A: not applicable information.
The charitable donations task performed during fMRI lasted for 144 rounds (3 runs; 48 rounds in each run) with the following conditions presented in pseudorandom order:
Costly donation: Charity gains, participant loses
Non-costly donation: Charity gains, participant neither gains nor loses
Reinforcing donation: Charity gains, participant gains
Simple financial reward: Participant gains, charity neither gains nor loses
Neutral: No gain or loss for either charity or participant.
See Table 1 for cost, donation and reward magnitudes in each condition. In total, there were three donation conditions where the charity gains: reinforcing donation (participant also gains), non-costly donation (no change for participant) and costly donation (cost/loss for participant). The costly condition best models real-life charitable giving. Donations in the costly proposals reduced participants' endowment by 30p (p = pence, 1/100th of £1, 1 UK pound), which is then escalated in the experimental design of the study (as in Moll et al. 2006) and corresponded to £1 of donation to the charity.
During the experiment, participants started with £20 of funds corresponding to real currency. In each round, charity information was presented to the participants, comprising the name of the charity and a shortened version of its mission statement (for 6 s). On the next screen, the participants saw the payoff conditions (for 3.5 s). Participants responded by using the designated “Accept” and “Reject” buttons to indicate their decisions; the designation of these two buttons was counterbalanced across participants. A 20p penalty was imposed when participants failed to respond within 3.5 s. The payoff screen remained visible until 3.5 s expired, irrespective of how quickly participants responded to the proposal. On the final screen, participants were presented with the outcome of their decisions and the amount of remaining funds (for 2.5 s; see Fig. 1 for the sequence of screens on experiment timeline). At the end of the game, the amount of remaining funds was rounded to the nearest lb to be received as reimbursement for participation. The participants were told that all of the donated money would be distributed evenly to the five most frequently selected charities once the study was completed, and in a debriefing session, no participant questioned whether these donations would be made.
Fig. 1.
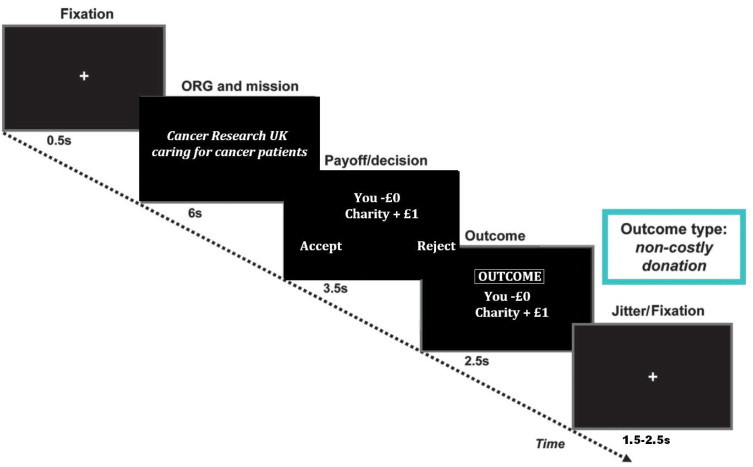
Diagram showing the experimental timeline of the charitable donations paradigm.
Adapted from Moll et al. (2006).
2.4. Image acquisition
Echo-planar T2*-weighted images (351 volumes in each of the 3 runs with 5 dummy scans for each run of 11 min 42 s) were acquired on a Philips 3 Tesla Achieva MRI scanner with an 8 channel coil, 3 mm slice thickness and ascending continuous acquisition parallel to the anterior to posterior commissural line (between 35 and 40 slices depending on size of the participant's head, Repetition Time (TR) = 2000 ms, Echo Time (TE) = 20.5 ms, Field of View (FOV) = 220 × 220 × 120 mm, acquisition matrix = 80 × 80, reconstructed voxel size = 2.29 × 2.29 × 3 mm, SENSE factor = 2) optimised for signal detection in ventral frontal areas (Green et al., 2012b). In addition 3-dimensional T1-weighted Magnetisation-Prepared Rapid Acquisition Gradient Echo structural images were obtained (reconstructed voxel size = 1 mm3, 128 slices, TE = 3.9 ms, FOV = 256 × 256 × 128, acquisition matrix = 256 × 164, slice thickness = 1 mm, TR = 9.4 ms). Axial T2-weighted structural images were acquired for each participant to rule out vascular and inflammatory abnormalities.
2.5. fMRI modelling
We modelled the haemodynamic response function with time and dispersion derivatives. The five condition specific regressors in the general linear model were: neutral (neither the participant nor the charity gains or loses money), costly (charity gains, participant loses), non-costly (charity gains, no change for participant) and reinforcing (charity and participant both gain) donation conditions and simple financial reward (participant gains). The baseline fixation condition was modelled explicitly as the sixth regressor, in order to replicate the analysis approach used by Moll et al. (2006). The regressors in the model referred to onset time vectors for all proposals that were accepted (and fixation onset for the baseline). Our event-related fMRI paradigm is modelled in the same way as Moll et al. (2006). In summary, we modelled the 3.5 s corresponding to the presentation of the proposal (payoff/decision screen in Fig. 1), during which participants made their decisions. This is the “decision phase” analysis. We also modelled the 6 s window containing both the payoff/decision and outcome screens (see Fig. 1) to detect outcome related activations. This is the “outcome phase” analysis. Since the outcome is fully predictable if the payoff is accepted, the outcome is known from the point at which the proposal is presented and accepted and therefore it makes sense to model this phase including the decision screen, following the approach of Moll et al. (2006).
2.6. Analysis
Behavioural and supporting data analyses were performed using a significance threshold of p = 0.05, 2-sided; using chi-square, independent sample t-tests and general linear models (SPSS 20.0, http://www.spss.com). Functional images were realigned, unwarped and coregistered to the subject's T1 images. These images were normalised by first normalising the participant's T1 image to the standard T1-template in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) and applying the same transformations to the functional images. A Gaussian kernel of 5 mm at full width at half maximum (FWHM) was used for smoothing to be sensitive to small subcortical areas of activation (Sacchet and Knutson, 2012). At the first (individual) level we contrasted donation (containing all accepted costly, non-costly and reinforcing donation proposals) vs. reward (simple financial reward) in a balanced contrast. Subsequently, we contrasted these proposal options in pairwise comparisons (e.g. costly vs. non-costly, costly vs. reward, non-costly vs. reward) and contrasted each proposal condition against the baseline conditions of neutral and fixation. At the second level, we used the contrast images from pairwise comparisons in two different random effects models. Using one-sample t-tests in our first model, we assessed neural differences between conditions separately in healthy subjects (n = 15) and in remitted patients (n = 14). Using a two-sample t-test in our second model we compared the groups. In secondary data analyses based on the peak-voxels of the whole brain between-group comparison models (using a 1.5 mm radius around the peak voxel in MarsBar version 0.43, http://marsbar.sourceforge.net/ (Brett et al., 2002)), we aimed to confirm that the detected regions survived when comparing donation/reward proposals vs. the low-level fixation condition, allowing us to infer either increased activation for the acceptance of the proposal or deactivation in the subtracted control condition.
Whole brain results were first explored at a voxel-level threshold of p = 0.005 uncorrected, cluster threshold 4 voxels. However, areas are only reported that survived additional voxel- or cluster-level Family-Wise-Error (FWE)-corrected thresholds of p = 0.05 across a priori ROIs (as detailed below, small volume correction) or the whole brain.
2.7. Region of interest (ROI) definition
We defined independent structural regions of interest which were previously shown to be involved in decisions to make charitable donations and are also critically associated with depression (septal/subgenual cingulate region and bilateral striatum). In an exploratory fashion, we also investigated the activations in two additional regions which are associated with social economical decision making; dorsal anterior cingulate and ventromedial prefrontal cortex (Pulcu et al., 2013). The ROIs were defined by using the Wake Forest University (WFU) PickAtlas tool (Maldjian et al., 2003) for SPM8; using a combination of anatomical and Brodmann Area masks from the automated anatomical labels (see Supplementary methods for the details).
3. Results
3.1. Participants
The groups did not differ significantly for age, years of education, distribution of gender, annual income or MADRS scores (see Table 2).
Table 2.
Group comparison of demographic and basic clinical variables (mean ± SD).
| Control | Remitted MDD | Test statistic | p-Value | |
|---|---|---|---|---|
| Age | 38.9 ± 5.9 | 38.1 ± 6.3 | 0.346T | 0.732 |
| Education (years) | 17.5 ± 4.1 | 16.8 ± 3.5 | 0.548T | 0.588 |
| Gender | 9 females | 12 females | 0.895a | 0.344 |
| Income (GBP) | 23,835 ± 8752 | 22,173 ± 9521 | 0.488T | 0.629 |
| MADRS | 2.35 ± 2.17 | 2.6 ± 2.9 | -0.254T | 0.801 |
| GAF | 91 ± 4.1 | 87.6 ± 5.9 | 1.968T | 0.059 |
T Denotes t-test.
Pearson's chi-square (df = 1). T= t-test. Control: N = 15, remitted MDD: N = 14. Patients with remitted major depression had a trend towards lower GAF scores compared with healthy subjects.
3.2. Behavioural results
There were no differences between groups in the number of acceptance responses to any of the donation or the reward conditions or response times for acceptance or rejection in any of the conditions (see Table 3). Furthermore, the groups did not differ on how they rated the mission statements of the charitable organisations and how much they were familiar with the charitable organisations prior to their participation.
Table 3.
Behavioural measures and response time (RT) comparisons (ms).
| Control (mean ± SD) | Remitted MDD (mean ± SD) | Ta | p-Values | |
|---|---|---|---|---|
| Donation count | ||||
| Costly | 18.9 ± 9.4 | 22.5 ± 10.7 | -0.943 | 0.354 |
| Non-costly | 30.9 ± 6.7 | 33.6 ± 4.5 | -1.270 | 0.215 |
| Rewarding | 22.3 ± 3.1 | 23.3 ± 1.8 | -1.104 | 0.28 |
| Simple financial reward | 21.1 ± 3.6 | 22 ± 2.1 | -0.794 | 0.434 |
| Neutral | 13.6 ± 9.5 | 16.3 ± 9.4 | -0.769 | 0.449 |
| Total funds (£) | 18 ± 2.4 | 17.6 ± 3.1 | 0.351 | 0.729 |
| Charity ratings | ||||
| Familiarity | 3.05 ± 0.9 | 2.8 ± 0.6 | 0.741 | 0.465 |
| Mission statement | 5.3 ± 0.7 | 5.2 ± 0.5 | 0.401 | 0.692 |
| Costly donation | ||||
| Acceptance RT | 1604 ± 311 | 1562 ± 420 | 0.307 | 0.761 |
| Rejection RT | 1567 ± 396 | 1608 ± 418 | -0.269 | 0.79 |
| Non-costly donation | ||||
| Acceptance RT | 1338 ± 329 | 1454 ± 557 | -0.677 | 0.504 |
| Rejection RT | 1406 ± 523 | 1639 ± 579 | -1.129 | 0.269 |
| Rewarding donation | ||||
| Acceptance RT | 1394 ± 522 | 1318 ± 392 | 0.445 | 0.66 |
| Rejection RT | 1710 ± 708 | 1475 ± 446 | 1.074 | 0.292 |
| Simple financial reward | ||||
| Acceptance RT | 1366 ± 530 | 1608 ± 545 | -1.209 | 0.237 |
| Rejection RT | 1452 ± 584 | 1476 ± 406 | -0.128 | 0.899 |
The groups do not differ on acceptance of donation proposals or any of the response times (control group: N = 15, rMDD group: N = 14).
Independent sample t-test (df = 27).
3.3. fMRI results
Summary of all fMRI activations is reported in Table 4.
Table 4.
Summary of BOLD fMRI results.
| Comparison | Hemisphere | Region | MNI | t-Value | FWE-corr.p-value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Controls | |||||||
| Donation + reward > fix | L | Ventromedial PFC | -9 | 47 | -14 | 6.74 | .01c, ROI |
| L | Septal | -3 | 23 | -2 | 4.08 | .02c, ROI | |
| L | Subgenual cingulate | -12 | 29 | -8 | 5.36 | .02c, ROI | |
| Donation > fix | R | Frontopolar cortex | 6 | 65 | -5 | 5.97 | <.001c, ROI |
| R | Lateral OFC | 21 | 35 | 4 | 4.99 | .02c, ROI | |
| L | Septal | -3 | 23 | -2 | 3.90 | .04c, ROI | |
| Reward > fix | L | Ventromedial PFC | -9 | 47 | -14 | 8.04 | <.001wb |
| L | Temporal gyrus | -63 | -7 | -14 | 8.07 | .001wb | |
| R | temporal gyrus | 63 | -4 | 7 | 5.78 | <.001wb | |
| R | Inferior frontal gyrus | 42 | 29 | -14 | 4.73 | .04wb | |
| R | Subgenual cingulate | 15 | 29 | -8 | 4.49 | .05wb | |
| R | Septal/nucleus accumbens | 3 | 20 | 4 | 4.74 | .02c, ROI | |
| Reward > costly | L | Dorsal ACC | -6 | 32 | 7 | 5.6 | .03c, ROI |
| Remitted MDD | |||||||
| Donation + reward > fix | R | Subgenual cingulate | 12 | 35 | -14 | 6.82 | .001c, ROI |
| L | Septal | -3 | 17 | -5 | 4.41 | .02c, ROI | |
| R | Striatum | 30 | -4 | -11 | 5.54 | .03c, ROI | |
| Donation > fix | R | Subgenual cingulate | 12 | 35 | -14 | 8.29 | .005wb |
| R | Striatum | 30 | -4 | -11 | 5.57 | .04c, ROI | |
| R | Head of caudate | 15 | 23 | 13 | 5.56 | .05c,ROI | |
| L | Septal/nucleus accumbens | -6 | 17 | -8 | 5.58 | .01c, ROI | |
| Control vs. rMDD | |||||||
| Costly > reward | R | Dorsal ACC | 15 | 26 | 16 | 4.4 | .05v, ROI |
| rMDD vs. control | |||||||
| Donation + reward > fix | R | Striatum | 18 | 20 | 1 | 3.94 | .05v, ROI |
| Donation > fix | R | Nucleus accumbens | 21 | 14 | -14 | 4.73 | .04v, ROI |
| Donation > reward | L | Subgenual cingulate | -3 | 29 | 1 | 4.1 | .04v, ROI |
Only regions that survived voxel- or cluster-based FWE-corrected p = .05 over the whole brain or our a priori ROIs are reported. wb = whole brain, c = cluster-based, v = voxel-based FWE-correction. Control group: N = 15, remitted MDD group: N = 14. fix: Fixation cross.
3.3.1. Healthy subjects
3.3.1.1. Decision phase. Against baseline fixation, decisions to donate were associated with increased BOLD response in the frontopolar and lateral orbitofrontal cortices and the septal region. When comparing decisions to accept simple rewards vs. making costly donations, healthy subjects showed an enhanced response in the dorsal anterior cingulate cortex (see Table 4). The comparisons between other main conditions in pairwise contrasts (i.e. all donation vs. reward, non-costly vs. reward, costly vs. non-costly) showed no significantly different responses.
3.3.1.2. Outcome phase. Outcomes in all donation and reward conditions against the low-level fixation baseline (i.e. all three donation conditions + reward > fixation) in healthy subjects were associated with increased responses in the sgACC, septal region and ventromedial prefrontal cortex, whereas outcomes for accepting simple rewards were associated with the medial temporal gyri bilaterally, inferior frontal gyrus, ventromedial PFC and septal/nucleus accumbens region and subgenual cingulate cortex.
3.3.2. Patients with remitted MDD (rMDD)
3.3.2.1. Decision phase. Increased BOLD response related to decisions to make charitable decisions (irrespective of personal costs) to accepting simple monetary rewards (i.e. all donation > reward contrast); as well as direct comparison of costly donations to accepting simple monetary rewards did not reach FWE-corrected significance level.
3.3.2.2. Outcome phase. Significantly increased BOLD response related to outcomes increasing the charity's and one's own financial resources against the baseline condition (i.e. all three donation conditions + reward > fixation, see Table 4) was detected in the sgACC. The inferior aspect of the globus pallidus within the right striatum, as well as the septal region, also showed increased response. Outcomes related to making donations relative to the baseline fixation cross were associated with BOLD response in the sgACC, striatum, head of caudate and septal/nucleus accumbens regions. Outcomes related to accepting simple financial rewards relative to the baseline fixation did not produce any significant change in BOLD response at FWE-corrected threshold.
3.3.3. Between group comparisons
3.3.3.1. Decision phase. When comparing all three donation decisions to simple rewards, there was increased sgACC response in the rMDD group relative to control subjects (BA 24; MNI: -3, 29, 1; t = 4.1, FWE corrected over ROI: p = 0.04; see Fig. 2). When comparing decisions to make costly donation vs. reward, there was increased dorsal anterior cingulate cortex response in control subjects relative to the rMDD group (BA 31; MNI: 15, 26, 16; t = 4.4, FWE corrected over ROI: p = 0.05; see Fig. 2).
Fig. 2.
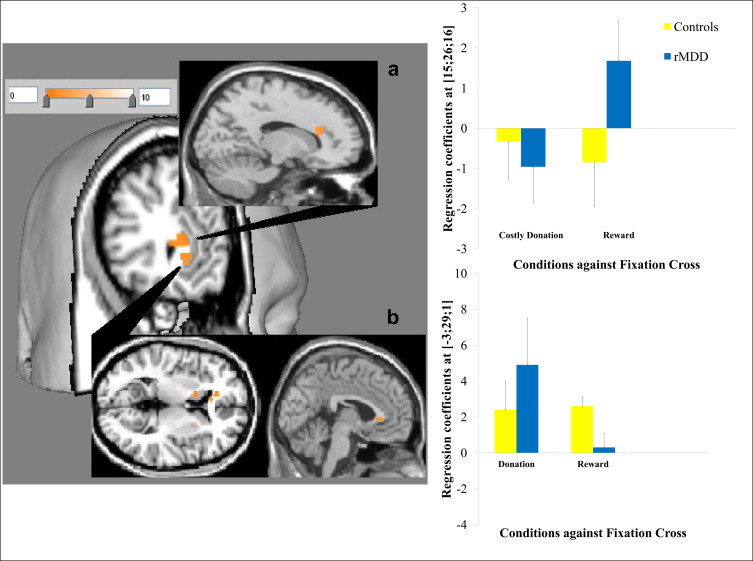
3D rendering and overlays constructed by Mango brain imaging software. T-maps displayed at p < 0.005 uncorrected showing activation in (a) the dorsal anterior cingulate in healthy subjects relative to MDD for costly donation > reward with regression coefficients from the peak voxel for the contrast elements against the fixation shown in bar charts (as with for other activations in the figures; error bars show ±1 standard error) and (b) the subgenual cingulate in MDD relative to healthy subjects for all donation > reward.
3.3.3.2. Outcome phase. Finally, there was increased striatum response in the rMDD group relative to control subjects when comparing all reward-related outcomes to fixation (i.e. all donation + reward > fixation; MNI: 18, 20, 1; t = 3.94, FWE corrected over ROI: p = 0.05; see Fig. 3). Enhanced donation specific response (i.e. all donation conditions > fixation) in the remitted group, relative to healthy subjects, was further confirmed by post-hoc SPM analysis showing a significant activation in the nucleus accumbens extending medially to the septal region adjacent to the sgACC (MNI: 21, 14, -14, t = 4.73, p = 0.04).
Fig. 3.
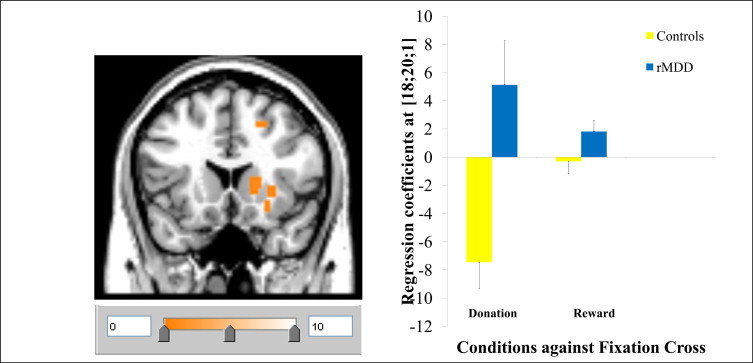
Activation in the right striatum in MDD relative to healthy subjects for all donation + reward > fixation.
Between group comparisons for other main contrasts (i.e. non-costly vs. reward; costly vs. non-costly) did not reveal any significant response differences in our ROIs in either the decision or outcome phases.
3.3.4. Supporting general linear models
The between group differences that we present above reflect group by condition interactions. In order to confirm group by condition interactions we used extracted regression coefficients from the peak voxels for the conditions of interest compared to baseline in 2 × 2 GLMs in SPSS (two types of decisions (e.g. to donate or to accept simple rewards) by two levels of clinical grouping). This subsequent analysis provides a useful check for the robustness of between group differences as SPM minimises the residual error over the whole brain (i.e. least-squares fitting) in GLMs, whereas using regression coefficients extracted by MarsBar in SPSS allows us to test the suitability of these whole brain models to specific regions. In order to understand the simple effects driving between group interactions, we did pairwise comparisons using extracted regression coefficients from the peak voxels for the conditions of interest compared to baseline (see between group differences presented in Figs. 2 and 3).
For the sgACC response in the decision phase (all donation > reward), there was a significant decision type by group interaction (F(1, 24) = 9.694, p = 0.005) with main effect of decisions (F(1, 24) = 4.944, p = 0.036), but no main effect of clinical group (p = 0.408). Pairwise comparisons were used to explore the interaction. Relative to healthy subjects, there was a significantly reduced response for receiving simple rewards (t = 2.295, p = 0.05), and marginally elevated response to making donations (t = -1.796, p = 0.09) in remitted patients. The magnitude of donation related activation relative to simple rewards was significantly higher in remitted patients (t = 3.887 p = 0.001), whereas it was comparable in healthy subjects (t = -0.123, p = 0.903; see Fig. 2b).
For the dACC response in the decision phase (costly donation > reward), there was a significant decision type by clinical group interaction (F(1, 24) = 10.336, p = 0.004) with main effect of decision type (F(1, 24) = 5.686, p = 0.025), but no main effect of clinical group (p = 0.390). Between group pairwise comparisons for simple main effects suggested that the groups were marginally different for reward related responses (t = -1.757, p = 0.09). Relative to the magnitude of responses for simple rewards, remitted patients had significantly lower response for costly donations (t = -2.182, p = 0.04), whereas in healthy subjects the magnitude of the deactivations for both conditions was comparable (t = 0.372, p = 0.713; see Fig. 2a).
For the right striatal response in the outcome phase (all donation + reward > fixation), there was a significant type of outcome by clinical group interaction (F(1, 24) = 13.159, p = 0.002) with main effect of outcome type (F(1, 24) = 6.410, p = 0.02) and main effect of clinical group (F(1, 24) = 16.763, p = 0.0006). Between group pairwise comparisons suggested that the interaction is driven by significantly elevated responses for donation conditions (t = -4.161, p = 0.001), but between group differences were non-significant for simple rewards (t = -1.875, p = 0.08). The right striatal activations for both conditions relative to the baseline were comparable in remitted patients (t = 1.029, p = 0.315), but in healthy subjects the deactivations were significantly greater for donation conditions (t = -3.476, p = 0.002; see Fig. 3).
3.3.5. Exploratory correlation analysis
In order to explore further the dACC response during the decision phase, we investigated the correlations between the regression coefficients and behavioural measures related to the decisions: response times and frequency for accepting donation or reward proposals. There was a positive correlation between dACC regression coefficients for reward > fixation and response times for decisions to accept simple financial rewards in the rMDD group (df = 13, r = 0.573, p = 0.041); whereas an inverse correlation emerged between this measure and the number of simple financial rewards accepted in healthy subjects (df = 14, r = -0.634, p = 0.011; p-values uncorrected; see Fig. 2a).
4. Discussion
Here, we conducted a functional neuroimaging study using an experimental charitable donations paradigm in order to investigate the neural basis of altruistic decisions in patients with remitted MDD. Our behavioural results for costly donation and accepting simple financial rewards (see Table 3) suggested that the experimental task effectively probed donation related financial decision-making; with all participants prepared to make costly donations even though these decisions reduced their overall gains from the task. We showed that during periods of remission, patients with MDD did not engage in altruistic decisions significantly more frequently than healthy subjects. However, our imaging results indicate that altruistic decisions are associated with enhanced septal/sgACC response in remitted MDD compared to controls. We also showed that outcome related striatal responses are relatively enhanced in rMDD compared to controls for both charitable and self-serving decisions. Finally, we showed that in remitted patients there is enhanced response in dACC for decisions to accept simple financial rewards, and a decreased response (i.e. negative BOLD effect) in this region for decisions to make costly donations.
We were only partially able to reproduce the findings of a previous donation study in healthy volunteers (Moll et al., 2006). We showed that in healthy subjects charitable decisions, as well as accepting simple financial rewards, activated overlapping neural circuitry with the Moll et al. (2006) study, but condition specific activations were only detected against the baseline condition. These differences may be due to the differences in charitable organisations used across the two experimental paradigms, and in particular to the differences in the nature of charitable giving between the United States and the United Kingdom (Wright, 2002). One key difference in the nature of charitable giving between these two countries is that in the US, donations contribute to tax relief (Harbaugh et al., 2007) and therefore there may be non-altruistic incentives to donate. In the UK, only higher earners, who pay income tax at a higher rate, receive tax relief on donations and none of our participants reported annual incomes that would include them in this tax bracket. Also, Wright (2002) argued that charitable giving is often a form of political expression in the US, where controversial mission objectives of many charitable organisations are likely to divide public opinion (as reflected in the subset of charities which probed opposition behaviour in Moll et al. (2006)). There are far fewer controversial charitable organisations in the UK and we were therefore unable to examine opposition. This difference in the nature of the charities may also explain why we did not replicate all of the previously reported results.
Our core hypotheses concerned differential responses between individuals with rMDD and healthy controls. As predicted, we showed increased response in the sgACC region for decisions to donate relative to simple monetary rewards which distinguished the rMDD from the control group. Abnormal functioning of the sgACC in MDD is well established in clinical research. Following volumetric correction for the reduction in grey matter, an abnormal hypermetabolism in the sgACC has been shown in current depression (Drevets and Savitz, 2008), which normalises upon remission from symptoms (Mayberg et al., 2000). Here we observed enhanced sgACC response during remission, in response to a specific cognitive challenge, suggesting some residual hypersensitivity which can be elicited with a suitable task probe. It is important to point out that the peak activation in the sgACC that we report in our between groups comparison is almost precisely overlapping with the peak of functional abnormality reported by Drevets et al. (1997).
Previous imaging studies have implicated the sgACC in prosocial decision-making, affiliative feelings and moral emotions, such as interpersonal (Zahn et al., 2009b) and altruistic guilt (Basile et al., 2011b), empathic concern for others (Zahn et al., 2009a) and compassion for other's psychological pain (Immordino-Yang et al., 2009). Lesions to ventromedial parts of the prefrontal cortex (Koenigs and Tranel, 2007) have shown to influence prosocial decisions negatively in interpersonal financial exchange. More specifically, septal neurodegeneration has been associated with a lack of affiliative feelings such as guilt and pity (Moll et al., 2011), suggesting that these prosocial emotions are important for balancing selfish motives in social economical decision making situations. Our charitable donations paradigm elicited enhanced sgACC response in remitted MDD, suggesting that donation may be a more sensitive probe of sgACC function in this group than paradigms exploring interpersonal guilt (Green et al., 2012b), which did not lead to between group BOLD signal differences in the sgACC. Given the literature relating sgACC to prosocial, affiliative and moral emotions, it is reasonable to suggest that such feelings are more strongly elicited in people with remitted MDD than controls during charitable donations. More specifically it has been argued that forms of guilt, particularly survivor guilt, may be important motivations for making charitable donations. It is therefore possible that the enhanced septal/sgACC response during donations in patients with rMDD reflects the higher levels of survivor guilt that have been suggested to underpin hyper-altruism in this group (O'Connor, 2012). However while this is a reasonable hypothesis arising from our present findings, it requires direct testing in future studies, as guilt is only one component of social decision-making which a donations task potentially probes.
Counter to our hypothesis, we observed enhanced signal in the striatum in remitted MDD, particularly for donation outcomes. Meta-analytical reviews show that the striatum responds during the prediction and consumption of salient rewards (Diekhof et al., 2012), whether primary or secondary (Sescousse et al., 2013). The striatal responses observed in the striatum in healthy controls were lower than we expected. This may reflect the relatively passive nature of the task, as previous studies indicated that competing for, or winning, financial rewards under conditions of uncertainty is associated with greater striatal responses relative to passive receipt of simple rewards (Elliott et al., 2000). Another explanation for the relatively small magnitude of striatal activations in our control group may be that the magnitude of our simple rewards was much smaller than that used by Moll et al. (2006) (see Table 1 and legends for detailed comparison). Cultural and task specific differences between the present study and Moll et al. (2006) may also be important factors. However, these arguments do not explain the relatively increased striatal responses observed in the remitted group. Previous studies have suggested reduced striatal responses to financial rewards in people with MDD, which may persist into remission (Tremblay et al., 2005; Schlaepfer et al., 2008; Knutson et al., 2008; Eshel and Roiser, 2010). Our findings suggest that decisions to make charitable donations enhance striatal responses in rMDD, perhaps reflecting enhanced sensitivity to social rewards. However, once again, this is a hypothesis that requires testing in future studies.
Despite relative paucity of directly comparable evidence in the literature, here we present novel findings of enhanced response to charitable decisions in our a priori ROIs (sgACC and striatum) detected in the absence of behavioural differences. These present findings add to a growing number of studies showing evidence for abnormal social perception and social emotion processing even in periods of stable remission. For example, previous studies from our research group showed that patients with remitted depression have disrupted functional connectivity between the sgACC and the right anterior temporal lobe for guilt (Green et al., 2012b) and enhanced BOLD response in the right amygdala for shame while rating unpleasantness of hypothetical social scenarios (Pulcu et al., 2014). Similarly, we showed that remitted patients exhibited attenuated medial prefrontal responses to positive social interaction images (Elliott et al., 2012). In all cases, these neuronal differences were observed in the absence of any differences in performance. One possible explanation of this is that the same behavioural output is being achieved using different mechanisms, in line with theories discussing “neuronal compensation” in pathology (Gramsbergen, 2007; Erk et al., 2011). However, in the context of social and emotional tasks it is also possible that the mechanisms mediating behavioural output are comparable but the neuronal differences we observe reflect differences in the feelings elicited by the same category of decisions. For example, it is possible that the patients make comparable decisions yet experience different emotions associated with these decisions. These neuronal differences may relate to a neurobiological basis of vulnerability, as people with history of a major depressive episode have higher susceptibility to further episodes (Eaton et al., 2008). Social reward hypersensitivity, observed at the neuronal level, in the remitted depression group may therefore reflect a trait vulnerability. Longitudinal neuroimaging studies of social cognition/decision-making could reveal more about the role of such abnormal neural responses in depression vulnerability.
Finally, we showed between-group differences for costly donations relative to accepting simple financial rewards in the dACC. Response in the dACC has been consistently demonstrated in paradigms using economical decision making, including activations related to making costly donations relative to accepting simple rewards as shown by Moll et al. (2006). Previous studies have also observed dACC responses when participants chose between outcomes in social and economic exchange contexts (Sanfey et al., 2003; Rilling et al., 2008). In our study, healthy subjects showed deactivations in this region for both conditions (i.e. costly donations and simple rewards), but more strongly for accepting simple rewards, whereas in the remitted depression group there was a selectively enhanced response when increasing their own payoff. There were no behavioural differences between patients and controls in terms of decisions for making costly donations or accepting simple financial rewards. However, healthy subjects with a greater magnitude of dACC deactivation for accepting simple rewards chose to accept them more frequently, whereas remitted patients who had stronger activations in this region took more time to make self-serving decisions (see Fig. 2a). A number of functional roles that have previously been ascribed to the dACC may be relevant to our task, including conflict resolution and action monitoring (Botvinick et al., 2004; Amodio and Frith, 2006), switching between different decision-making modes (Rushworth et al., 2007) or evaluation of the anticipated reward utility of outcomes (Walton et al., 2007). However our task was not explicitly designed to evaluate these mechanisms and thus no weight can be attached to such post-hoc explanations based on reverse inference.
Our study had a number of limitations. Like other key publications on neurobiology of donation behaviour (Moll et al., 2006; Harbaugh et al., 2007; Izuma et al., 2010), we did not correct our p-values for the number of exploratory ROIs that we used. It is also important to acknowledge our relatively small sample size in the present study. Despite showing donation-related hyperactivation in the group with MDD vulnerability, our study cannot discriminate between primary vulnerability (e.g. familial history) and secondary vulnerability (e.g. due to a previous episode). This issue should be addressed by longitudinal studies which also recruit individuals before the first onset of MDD. Finally, it is important to note that the patients in this cohort were fully remitted at the time of testing, and that remission was particularly stable in this cohort (mean of nearly 5 years). It is possible that such a stably remitted group of patients is not representative and it would be important to extend these findings to more recently remitted, and indeed currently depressed participants.
5. Conclusions
Here, we showed that there is a hyperactivation of septal/sgACC and striatum during charitable donation decisions in patients with MDD. Our findings suggest that a charitable donations paradigm may be a particularly sensitive probe of fronto-meso-limbic circuitry in depression, associated with neuronal abnormalities even in very stable remission. We suggest that the between-group differences that we demonstrate here related to social reward hypersensitivity may be related to biological trait markers for vulnerability to MDD for patients in stable remission.
Acknowledgements
This study was funded by Medical Research Council, UK (grant G0900593). RZ was funded by Medical Research Council, UK (G0902304).
Appendix A. Supplementary materials
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.nicl.2014.04.010.
Appendix A. Supplementary materials
Supplementary materials Enhanced subgenual cingulate response to altruistic decisions in remitted major depressive disorder.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. fourth edition. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews. Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. 16552413 [DOI] [PubMed] [Google Scholar]
- Andreoni J. Impure altruism and donations to public goods: a theory of warm-glow giving. Economic Journal. 1990;100:464–477. [Google Scholar]
- Basile B., Mancini F., Macaluso E., Caltagirone C., Frackowiak R.S., Bozzali M. Deontological and altruistic guilt: evidence for distinct neurobiological substrates. Human Brain Mapping. 2011;32:229–239. doi: 10.1002/hbm.21009. 20842749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile B., Mancini F., Macaluso E., Caltagirone C., Frackowiak R.S.J., Bozzali M. Deontological and altruistic guilt: evidence for distinct neurobiological substrates. Human Brain Mapping. 2011;32:229–239. doi: 10.1002/hbm.21009. 20842749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z., Cowen P.J. ‘It's not over when it's over’: persistent neurobiological abnormalities in recovered depressed patients. Psychological Medicine. 2008;38:307–313. doi: 10.1017/s0033291707001250. 18444278 [DOI] [PubMed] [Google Scholar]
- Botteron K.N., Raichle M.E., Drevets W.C., Heath A.C., Todd R.D. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biological Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. 11958786 [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. 15556023 [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.-L., Valabregue R., Poline J.-B. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan; Academic Press; 2002. [Google Scholar]
- Dichter G.S., Kozink R.V., Mcclernon F.J., Smoski M.J. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. Journal of Affective Disorders. 2012;136:1126–1134. doi: 10.1016/j.jad.2011.09.048. 22036801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Kaps L., Falkai P., Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude—an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007. 22366111 [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Ongur D., Price J.L. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Molecular Psychiatry. 1998;3:220–226. doi: 10.1038/sj.mp.4000370. 9672897 [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Simpson J.R., Todd R.D., Reich T., Vannier M., Raichle M.E. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. 9126739 [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Savitz J. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13:663–681. doi: 10.1017/s1092852900013754. 18704022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W.W., Shao H., Nestadt G., Lee B.H., Bienvenu O.J., Zandi P. Population-based study of first onset and chronicity in major depressive disorder. Archives of General Psychiatry. 2008;65:513–520. doi: 10.1001/archpsyc.65.5.513. 18458203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N. Emotion, regulation, and moral development. Annual Review of Psychology. 2000;51:665–697. doi: 10.1146/annurev.psych.51.1.665. 10751984 [DOI] [PubMed] [Google Scholar]
- Elliott R., Friston K.J., Dolan R.J. Dissociable neural responses in human reward systems. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. 10934265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R., Lythe K., Lee R., Mckie S., Juhasz G., Thomas E.J., Downey D., Deakin J., Anderson I.M. Reduced medial prefrontal responses to social interaction images in remitted depression. Archives of General Psychiatry. 2012;69:37–45. doi: 10.1001/archgenpsychiatry.2011.139. 22213787 [DOI] [PubMed] [Google Scholar]
- Erk S., Spottke A., Meisen A., Wagner M., Walter H., Jessen F. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Archives of General Psychiatry. 2011;68:845–852. doi: 10.1001/archgenpsychiatry.2011.80. 21810648 [DOI] [PubMed] [Google Scholar]
- Eshel N., Roiser J.P. Reward and punishment processing in depression. Biological Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. 20303067 [DOI] [PubMed] [Google Scholar]
- Feldman Hall O., Dalgleish T., Thompson R., Evans D., Schweizer S., Mobbs D. Differential neural circuitry and self-interest in real vs hypothetical moral decisions. Social Cognitive and Affective Neuroscience. 2012;7:743–751. doi: 10.1093/scan/nss069. 22711879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Miriam G., Williams J.B.W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Research Version, Non-patient Edition (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Foster K.R., Wenseleers T., Ratnieks F.L. Kin selection is the key to altruism. Trends in Ecology & Evolution. 2006;21:57–60. doi: 10.1016/j.tree.2005.11.020. 16701471 [DOI] [PubMed] [Google Scholar]
- Fujiwara T. Is altruistic behavior associated with major depression onset? PloS One. 2009;4:e4557. doi: 10.1371/journal.pone.0004557. 19234611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsbergen A. Neural compensation after early lesions: a clinical view of animal experiments. Neuroscience and Biobehavioral Reviews. 2007;31:1088–1094. doi: 10.1016/j.neubiorev.2007.10.002. 18053874 [DOI] [PubMed] [Google Scholar]
- Green S., Lambon Ralph M.A., Moll J., Deakin J.F., Zahn R. Guilt-selective functional disconnection of anterior temporal and subgenual cortices in major depressive disorder. Archives of General Psychiatry. 2012;69:1014–1021. doi: 10.1001/archgenpsychiatry.2012.135. 22638494 [DOI] [PubMed] [Google Scholar]
- Green S., Matthew Lambon Ralph J.M.O.L.L., Deakin W., Zahn R. Guilt-selective functional disconnection of anterior temporal and subgenual cortices in major depressive disorder. Archives of General Psychiatry. 2012;173:1014–1021. doi: 10.1001/archgenpsychiatry.2012.135. 22638494 [DOI] [PubMed] [Google Scholar]
- Green S., Moll J., Deakin J.F.W., Hulleman J., Zahn R. Proneness to decreased negative emotions in major depressive disorder when blaming others rather than oneself. Psychopathology. 2013;46:34–44. doi: 10.1159/000338632. 22890331 [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., Glover G.H., Solvason H.B., Kenna H., Reiss A.L., Schatzberg A.F. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. 17210143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W.D. The evolution of altruistic behavior. The American Naturalist. 1963;97:354–356. [Google Scholar]
- Harbaugh W.T., Mayr U., Burghart D.R. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science (New York, N.Y.) 2007;316:1622–1625. doi: 10.1126/science.1140738. 17569866 [DOI] [PubMed] [Google Scholar]
- Hare T.A., Camerer C.F., Knoepfle D.T., O'Doherty J.P., Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2010;30:583–590. doi: 10.1523/JNEUROSCI.4089-09.2010. 20071521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M., Anen C., Quartz S.R. The right and the good: distributive justice and neural encoding of equity and efficiency. Science (New York, N.Y.) 2008;320:1092–1095. doi: 10.1126/science.1153651. 18467558 [DOI] [PubMed] [Google Scholar]
- Immordino-Yang M.H., Mccoll A., Damasio H., Damasio A. Neural correlates of admiration and compassion. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8021–8026. doi: 10.1073/pnas.0810363106. 19414310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K., Saito D.N., Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. Journal of Cognitive Neuroscience. 2010;22:621–631. doi: 10.1162/jocn.2009.21228. 19320552 [DOI] [PubMed] [Google Scholar]
- Kim S., Thibodeau R., Jorgensen R.S. Shame, guilt, and depressive symptoms: a meta-analytic review. Psychological Bulletin. 2011;137:68–96. doi: 10.1037/a0021466. 21219057 [DOI] [PubMed] [Google Scholar]
- Knutson B., Bhanji J.P., Cooney R.E., Atlas L.Y., Gotlib I.H. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. 17916330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the ultimatum game. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2007;27:951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. 17251437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmbeck J.T., Brassen S., Braus D.F., Weber-Fahr W. Subgenual anterior cingulate cortex alterations in late-onset depression are related to “pessimistic thoughts”. American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2008;16:248–249. doi: 10.1097/JGP.0b013e318162a0c0. 18310555 [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. 12880848 [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Brannan S.K., Mahurin R.K., Jerabek P.A., Brickman J.S., Tekell J.L., Silva J.A., Mcginnis S., Glass T.G., Martin C.C., Fox P.T. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. 9141092 [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Brannan S.K., Tekell J.L., Silva J.A., Mahurin R.K., Mcginnis S., Jerabek P.A. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biological Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. 11063978 [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Lozano A.M., Voon V., Mcneely H.E., Seminowicz D., Hamani C., Schwalb J.M., Kennedy S.H. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. 15748841 [DOI] [PubMed] [Google Scholar]
- Moll J., Krueger F., Zahn R., Pardini M., de Oliveira-Souzat R., Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. 17030808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J., Zahn R., de Oliveira-Souza R., Bramati I.E., Krueger F., Tura B., Cavanagh A.L., Grafman J. Impairment of prosocial sentiments is associated with frontopolar and septal damage in frontotemporal dementia. Neuroimage. 2011;54:1735–1742. doi: 10.1016/j.neuroimage.2010.08.026. 20728544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry: the Journal of Mental Science. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. 444788 [DOI] [PubMed] [Google Scholar]
- Morey R.A., Mccarthy G., Selgrade E.S., Seth S., Nasser J.D., Labar K.S. Neural systems for guilt from actions affecting self versus others. NeuroImage. 2012;60:683–692. doi: 10.1016/j.neuroimage.2011.12.069. 22230947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon N., Liddle P., Nixon E., Worwood G., Liotti M., Palaniyappan L. Biological vulnerability to depression: linked structural and functional brain network findings. British Journal of Psychiatry: the Journal of Mental Science. 2014;204:283–289. doi: 10.1192/bjp.bp.113.129965. 24357570 [DOI] [PubMed] [Google Scholar]
- O'Connor L.E., Berry J.W., Lewis T.B., Stiver D.J. Pathological Altruism. Oxford Scholarship Online; 2012. Empathy-based pathogenic guilt, pathological altruism, and psychopathology. [Google Scholar]
- O'Connor L.E., Berry J.W., Weiss J., Schweitzer D., Sevier M. Survivor guilt, submissive behaviour and evolutionary theory: the down-side of winning in social comparison. British Journal of Medical Psychology. 2000;73:519–530. doi: 10.1348/000711200160705. 11140792 [DOI] [PubMed] [Google Scholar]
- Pulcu E., Lythe K., Elliott R., Green S., Moll J., Deakin J.F., Zahn R. Increased amygdala response to shame in remitted major depressive disorder. PLoS ONE. 2014;9:e86900. doi: 10.1371/journal.pone.0086900. 24497992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulcu E., Zahn R., Elliott R. The role of self-blaming moral emotions in major depression and their impact on social-economical decision making. Frontiers in Psychology. 2013;4:1–17. doi: 10.3389/fpsyg.2013.00310. 23382719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., King-Casas B., Sanfey A.G. The neurobiology of social decision-making. Current Opinion in Neurobiology. 2008;18:159–165. doi: 10.1016/j.conb.2008.06.003. 18639633 [DOI] [PubMed] [Google Scholar]
- Rushworth M.F., Buckley M.J., Behrens T.E., Walton M.E., Bannerman D.M. Functional organization of the medial frontal cortex. Current Opinion in Neurobiology. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. 17350820 [DOI] [PubMed] [Google Scholar]
- Sacchet M.D., Knutson B. Spatial smoothing systematically biases the localization of reward-related brain activity. Neuroimage. 2012;66:270–277. doi: 10.1016/j.neuroimage.2012.10.056. 23110886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey A.G., Rilling J.K., Aronson J.A., Nystrom L.E., Cohen J.D. The neural basis of economic decision-making in the ultimatum game. Science (New York, N.Y.) 2003;300:1755–1758. doi: 10.1126/science.1082976. 12805551 [DOI] [PubMed] [Google Scholar]
- Schiller C.E., Minkel J., Smoski M.J., Dichter G.S. Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. Journal of Affective Disorders. 2013;151:756–762. doi: 10.1016/j.jad.2013.06.016. 23835103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer T.E., Cohen M.X., Frick C., Kosel M., Brodesser D., Axmacher N., Joe A.Y., Kreft M., Lenartz D., Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. 17429407 [DOI] [PubMed] [Google Scholar]
- Sescousse G., Caldú X., Segura B., Dreher J.-C. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2013;37:681–696. doi: 10.1016/j.neubiorev.2013.02.002. 23415703 [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. 9881538 [PubMed] [Google Scholar]
- Siegle G.J., Carter C.S., Thase M.E. Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. 16585452 [DOI] [PubMed] [Google Scholar]
- Tangney J.P., Stuewig J., Mashek D.J. Moral emotions and moral behavior. Annual Review of Psychology. 2007;58:345–372. doi: 10.1146/annurev.psych.56.091103.070145. 16953797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L.K., Naranjo C.A., Graham S.J., Herrmann N., Mayberg H.S., Hevenor S., Busto U.E. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Archives of General Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. 16275810 [DOI] [PubMed] [Google Scholar]
- Walton M.E., Croxson P.L., Behrens T.E., Kennerley S.W., Rushworth M.F. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage. 2007;36 Suppl 2:T142–T154. doi: 10.1016/j.neuroimage.2007.03.029. 17499161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. Generosity Versus Altruism: Philanthropy and Charity in the US and UK. Centre for Civil Society; 2002. [Google Scholar]
- Zahn R., de Oliveira-Souza R., Bramati I., Garrido G., Moll J. Subgenual cingulate activity reflects individual differences in empathic concern. Neuroscience Letters. 2009;457:107–110. doi: 10.1016/j.neulet.2009.03.090. 19429173 [DOI] [PubMed] [Google Scholar]
- Zahn R., Moll J., Paiva M., Garrido G., Krueger F., Huey E.D., Grafman J. The neural basis of human social values: evidence from functional MRI. Cerebral Cortex (New York, N.Y.: 1991) 2009;19:276–283. doi: 10.1093/cercor/bhn080. 18502730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R., Moll J., Paiva M.M.F., Garrido G., Krueger F., Huey E.D., Grafman J. The neural basis of human social values: evidence from functional MRI. Cerebral Cortex (New York, N.Y.: 1991) 2009;19:276–283. doi: 10.1093/cercor/bhn080. 18502730 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials Enhanced subgenual cingulate response to altruistic decisions in remitted major depressive disorder.


