FIG 2.
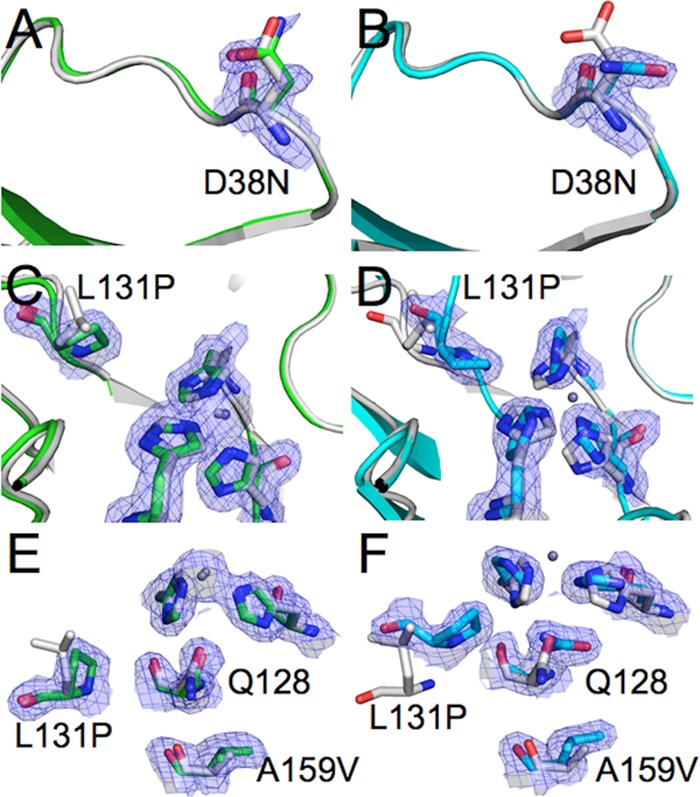
Effects of the mutations in holo-TrzN-G3 (green) (A, B, and C) and apo-TrzN-G3 (cyan) (D, E, and F). (A and D) The structure of native holo-TrzN is shown in gray for comparison. The D38N mutation results in little conformational change in the holo-TrzN-G3 structure, while an alternative rotamer is adopted in apo-TrzN-G3. (B and E) The L131P mutation results in an increase in the volume of the active-site binding pocket but no other backbone changes in holo-TrzN-G3, whereas in apo-TrzN-G3, the proline residue stabilizes a tight turn in the alternative backbone conformation. (C and F) The A159V mutation results in a slight increase in an already very small cavity in native holo-TrzN, whereas in the rearranged structure of apo-TrzN-G3, a much larger cavity is formed, which is filled by the A159V mutation.
