FIG 3.
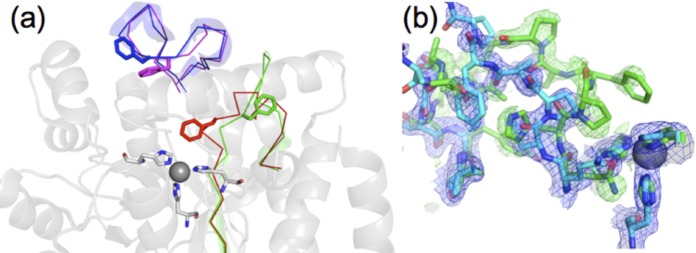
Comparison between apo-TrzN-G3 and holo-TrzN-G3. (a) Two loops undergo conformational change as a result of metal binding, with loop 2 (H130-Y141) in apo-TrzN-G3 (red) moving out of the active site in holo-TrzN-G3 (green). Loop 3 (S161-P180) undergoes a smaller change from apo-TrzN-G3 (magenta) to holo-TrzN-G3 (blue), which results in opening of the gorge to the active site. (b) Density from a single protein crystal structure in which the Zn2+ site is occupied at only 50%. The 2mfo-Dfc, αcalc map (blue) is contoured at 1σ, with the holo-TrzN-like chain model colored cyan (50% occupancy); the mfo-Dfc, αcalc map (green) is contoured at 2.5σ, demonstrating that the apo-TrzN-like chain (green; 50% occupancy) is also present in this structure. The second subunit of the dimer (subunit A) had 100% metal ion occupancy and no detectable density corresponding to apo-TrzN.
