FIG 4.
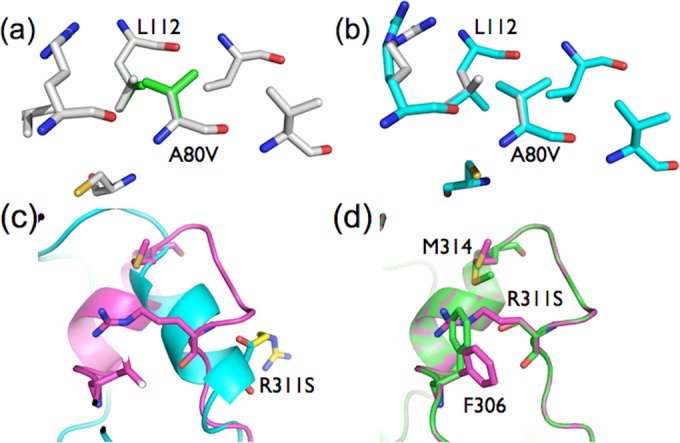
Effects of stabilizing mutations in holo-PTE and apo-PTE. (a) The A80V mutation (green) results in almost no conformational change in the holo-PTE structure (gray), only adding two methyl groups at the enzyme surface. (b) In contrast, in apo-PTE, the A80V mutation (cyan) is predicted to result in the adjacent leucine (L112) adopting a new rotamer, in which one of its methyl side chains fills a hydrophobic cavity below, which explains its predicted stabilizing effect on apo-PTE and increase in expression. (c) The R311S mutation in holo-PTE (cyan/yellow) results in the loss of a charged group on the protein surface. (d) In contrast, in apo-PTE, structural rearrangement of the apoeznyme results in R311 filling a cavity between F306 and M314. The R311S mutation allows F306 and M314 to collapse inward, filling the cavity and providing a stabilizing effect.
