Abstract
The Escherichia coli heat-labile enterotoxin B subunit (LTB) is a potent vaccine adjuvant. Salmonella enterica serovar Enteritidis ghosts carrying LTB (S. Enteritidis-LTB ghosts) were genetically constructed using a novel plasmid, pJHL187-LTB, designed for the coexpression of the LTB and E lysis proteins. S. Enteritidis-LTB ghosts were characterized using scanning electron microscopy to visualize their transmembrane tunnel structures. The expression of LTB in S. Enteritidis-LTB ghost preparations was confirmed by immunoblot and enzyme-linked immunosorbent assays. The parenteral adjuvant activity of LTB was demonstrated by immunizing chickens with either S. Enteritidis-LTB ghosts or S. Enteritidis ghosts. Chickens were intramuscularly primed at 5 weeks of age and subsequently boosted at 8 weeks of age. In total, 60 chickens were equally divided into three groups (n = 20 for each): group A, nonvaccinated control; group B, immunized with S. Enteritidis-LTB ghosts; and group C, immunized with S. Enteritidis ghosts. Compared with the nonimmunized chickens (group A), the immunized chickens (groups B and C) exhibited increased titers of plasma IgG and intestinal secretory IgA antibodies. The CD3+ CD4+ subpopulation of T cells was also significantly increased in both immunized groups. Among the immunized chickens, those in group B exhibited significantly increased titers of specific plasma IgG and intestinal secretory IgA (sIgA) antibodies compared with those in group C, indicating the immunomodulatory effects of the LTB adjuvant. Furthermore, both immunized groups exhibited decreased bacterial loads in their feces and internal organs. These results indicate that parenteral immunization with S. Enteritidis-LTB ghosts can stimulate superior induction of systemic and mucosal immune responses compared to immunization with S. Enteritidis ghosts alone, thus conferring efficient protection against salmonellosis.
INTRODUCTION
Salmonella enterica serovar Enteritidis, a Gram-negative intracellular pathogen, is frequently isolated from human infections (1). Salmonella infection exerts a considerable burden on both developing and developed countries; for example, the high prevalence of food-borne salmonellosis has been estimated to result in approximately 155,000 deaths worldwide every year (2). Infected poultry meat and eggs are the primary repositories for the strains of S. Enteritidis associated with human illness (3). S. Enteritidis-infected chickens do not show severe symptoms of infection; rather, they maintain a carrier state, which results in bird-to-bird spread of S. Enteritidis through vertical transmission and fecal shedding (4). The establishment of protective immunity by bird vaccination has been proposed as an ideal strategy for preventing S. Enteritidis infection on poultry farms (5).
The development of inactivated vaccines that are both safe and capable of inducing a specific and efficient immune response against S. Enteritidis is of utmost importance for protecting the health of both chickens and humans (5). Strategies for producing traditional killed vaccines involve the use of heat or chemical treatment to inactivate bacterial cells; however, these strategies can affect the physiochemical/structural properties of bacterial surface antigens and thus potentially inhibit the development of protective immunity (6). Traditional killed vaccines generally require a strong chemical adjuvant and several injections to induce suitable immunity, and they pose a greater risk of allergic reactions and vaccine injection site sarcomas (7). Genetic inactivation of Gram-negative bacteria by controlled expression of the cloned bacteriophage phi X174 E lysis gene offers a promising approach in inactivated vaccine technology to protection against infectious diseases (8). Since bacterial ghosts maintain the functional and antigenic envelope structures of their native live counterparts, these ghosts are capable of inducing strong humoral and cell-mediated immune responses. For example, Salmonella ghosts have been shown to induce protective immune responses in chickens (9–11).
The immunogenic potential of S. Enteritidis ghosts can be further enhanced by incorporating immunomodulatory molecules into the architecture of the ghosts themselves (12). The heat-labile enterotoxin of Escherichia coli (LT) is composed of a single A subunit and five identical B subunits (13). The B subunit is known to undergo stable cross-linking with the eukaryotic cell surface molecule, GM1; this high-affinity binding is thought to mediate its adjuvant activity (14). Since the LT B subunit (LTB) has the ability to bind target cells, it has been used as a carrier to enhance cellular uptake of genetically fused or physically linked antigens (12, 15, 16).
In this study, an asd+ ghost plasmid (pJHL187) harboring the E lysis gene cassette and a foreign antigen delivery cassette were used to produce S. Enteritidis ghosts. The regulatory E lysis ghost cassette was constructed using a convergent promoter design. To assess the parenteral adjuvant properties of LTB, the chickens were immunized with either S. Enteritidis-LTB ghosts or S. Enteritidis ghosts. The induction of immune responses and protective efficacies against virulent challenge were then assessed.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
Bacterial strains, plasmid vectors, and primers used in this study are listed in Table 1. All asd deletion strains of S. Enteritidis were grown at 37°C in LB broth containing 50 μg/ml diaminopimelic acid. Strains carrying ghost plasmids were propagated in the presence of l-arabinose. All bacterial strains were stored at −80°C in growth medium containing 20% glycerol.
TABLE 1.
Bacterial strains, plasmids, and primers utilized in this study
| Strain, plasmid, or primer | Description | Reference or source |
|---|---|---|
| Bacterial strains | ||
| E. coli | F− ompT hsdSB (rB− mB−) dcm gal λ(DE3) pLysS Cmr | Promega |
| BL21(DE3)pLysS | ||
| JOL500 | Wild-type F18+, LT+, STa+, STb+, stx2+, stx2e+ ETEC isolate from pig | Lab stock |
| S. Enteritidis | ||
| JOL1254 | asd gene knockout strain | Lab stock |
| JOL1182 | Wild type | Lab stock |
| JOL1373 | JOL1254 containing pMMP172 | Lab stock |
| JOL1358 | JOL1254 containing pJHL187-LTB | This study |
| JOL860 | Wild-type isolate from chickens | Lab stock |
| Plasmids | ||
| pMMP172 | asd+ pBR ori plasmid carrying ghost cassette | 19 |
| pET28a | IPTG-inducible expression vector; Kmr | Novagen |
| pET28a-LTB | pET28a derivative containing eltB | Present study |
| pYA3342 | asd+ vector, pBR ori | 44 |
| pYA3332 | asd+ vector; p15A ori | 44 |
| pJHL187 | asd+ p15A ori plasmid carrying ghost cassette | This study |
| pJHL187-LTB | pJHL187 containing eltB gene | This study |
| Primers | ||
| eltB-F | 5′-CCGCGAATTCGCTCCCCAGTCTATTACAG-3′ | 39 |
| eltB-R | 5′-CCGCAAGCTTCTAGTTTTCCATACTGATTG-3′ | |
| ompA-F-NcoI | 5′-CCATGGATGAAAAAGACAGCTATCGC-3′ | This study |
| OmpA-E/K/H-H6-Sal-R | 5′-TAAGTCGACATGATGATGATGATGATGAAGCTTGGTACCGAATTCCAGACGGGTAGCGAT-3′ |
Construction of plasmids carrying ghost cassettes and the antigen delivery system.
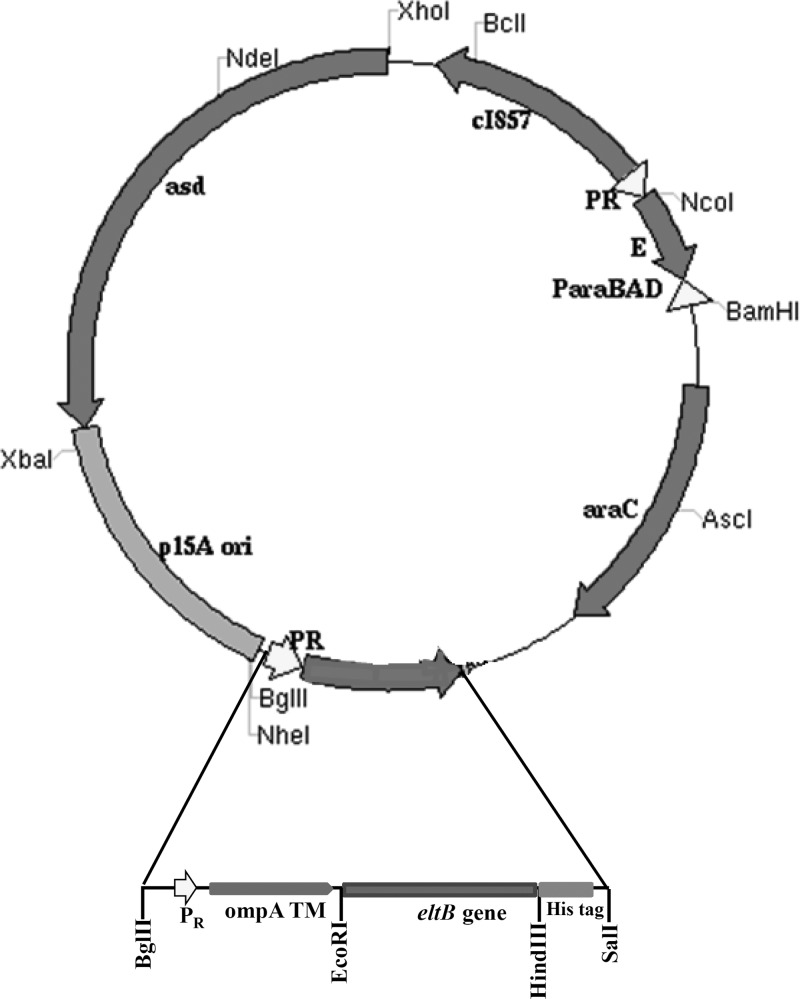
The regulatory E lysis ghost cassette was based on a convergent promoter construct, in which the E lysis gene was subcloned between a sense λpR promoter with a cI857 regulatory element and an antisense ParaBAD promoter with an araC regulatory element.
The backbone plasmid used to carry the regulatory E lysis cassette pYA3342 contained a pBR origin, a multicloning site (MCS), and the asd gene. The XbaI-BglII 1-kb fragment from pYA3342 carrying the ghost cassette was replaced with the XbaI-BglII fragment from pYA3332. A DNA segment of the ompA gene was PCR amplified by E. coli genomic DNA as a template. The primers used for cloning of the ompA gene are mentioned in Table 1. To construct the foreign antigen delivery system, the DNA sequence encoding the six transmembrane domains (TMD) out of an 8-TMD region from the E. coli outer membrane protein A (ompA) (17, 18) was placed under the control of the λpR promoter. The TMD region of ompA was then fused in-frame with the His6 epitope sequence, and the 3′ end of the His6 epitope sequence was ligated with the MCS, thus allowing the subcloning of foreign antigens. The resultant plasmid, harboring the p15A origin of replication, was designated pJHL187.
Subcloning eltB into the ghost plasmid.
The ghost plasmid harboring the tightly regulated E-mediated lysis cassette, pJHL187, was used to subclone the eltB sequence. The primers eltB-F (5′-CCGCGAATTCGCTCCCCAGTCTATTACAG-3′) and eltB-R (5′-CCGCAAGCTTCTAGTTTTCCATACTGATTG-3′) were used to amplify the eltB sequence by PCR from the genomic DNA of E. coli strain JOL500. The resultant PCR products were subcloned into the overexpression plasmid pET28a, thus generating pET28a-LTB. The E. coli BL21(DE3) pLysS strain was transformed with pET28a-LTB, and recombinantly produced LTB was purified using Ni-nitrilotriacetic acid (NTA) agarose (Qiagen, Valencia, CA). Protein purity was verified by Coomassie blue staining of SDS-polyacrylamide gel, and the total amount of purified protein was determined using the Bio-Rad protein assay kit, with bovine serum albumin used as a standard. The eltB gene fragment was isolated from pET28a-LTB by digestion with EcoRI and HindIII and subsequently subcloned into the MCS of pJHL187, thus placing it under the control of the λpR promoter. The resultant plasmid was designated pJHL187-LTB. The ghost plasmid pMMP172 is devoid of the foreign antigen delivery cassette (19) and was utilized as the vector control.
Preparation of anti-LTB rabbit serum.
The preparation of specific antibodies against the LTB protein was carried out via subcutaneous injection of an emulsion containing approximately 250 μg of purified LTB protein in 1 ml of sterile PBS and 1 ml of complete Freund's adjuvant into a New Zealand white rabbit. Two boosters with the same antigen quantity in incomplete Freund's adjuvant were administered at days 14 and 28 post-prime immunization. Blood was collected for the preparation of antisera on day 14 after final immunization.
Production and characterization of S. Enteritidis-LTB ghosts.
The S. Enteritidis asd knockout strain (JOL1254) was transformed with pJHL187-LTB, and the resultant strain was designated S. Enteritidis JOL1358. A single colony of JOL1358 was inoculated into nutrient broth containing 0.2% l-arabinose, and cultures were grown at 28°C until mid-logarithmic growth was reached. The cells were then collected, washed twice, resuspended in 100 ml nutrient broth without l-arabinose, and shifted to 42°C to induce the expression of LTB and E-mediated lysis. After 48 h, the ghost cells were harvested, washed twice with sterile phosphate-buffered saline (PBS) (pH 7.4), and stored at −70°C. For scanning electron microscopy (SEM) observations, S. Enteritidis ghosts were prepared as previously described (11). The JOL1254 strain was transformed with the vector control ghost plasmid pMMP172, and resultant strain was named S. Enteritidis JOL1373. A similar procedure was used to generate S. Enteritidis ghosts from JOL1373 cells.
Validation of LTB expression.
The total outer membrane protein fraction from the S. Enteritidis-LTB ghost was prepared as follows. Briefly, the S. Enteritidis-LTB ghost samples were subjected to sonication, and the suspension was subsequently centrifuged at 20,000 rpm for 30 min. The pellet was dissolved in Tris-Sarkosyl buffer (20 mM Tris containing 1% Sarkosyl [pH 8.6]) and incubated on ice for 30 min. The suspension was centrifuged at 132,000 × g for 1 h at 4°C to obtain the supernatant containing the outer membrane protein fraction. A similar procedure was used to prepare outer membrane protein fractions of S. Enteritidis ghost. Protein extracts of S. Enteritidis-LTB ghosts were heated at 95°C for 5 min and then resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 15% gels. The resolved proteins were then electrophoretically transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA) for immunoblotting. The membranes were blocked overnight at 4°C with 3% bovine serum albumin (BSA) in PBS with 0.01% Tween 20, and they were subsequently incubated with polyclonal anti-LTB rabbit serum and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibodies. Immunoreactive bands were detected with chemiluminescent dye and the WEST-one Western blot detection system (iNtRON, Seongnam, South Korea). Signals were detected using the multiwavelength illumination system on a Kodak Image Station 4000MM (Kodak, New Haven, CT). For enzyme-linked immunosorbent assay (ELISA)-mediated detection of LTB, S. Enteritidis-LTB ghosts were coated onto polystyrene ELISA plates and then blocked with 1% bovine serum albumin. The wells were then incubated with either polyclonal anti-LTB rabbit serum or rabbit serum lacking anti-LTB antibodies (1:5,000 dilution each) and subsequently incubated with goat anti-rabbit HRP-conjugated secondary antibodies. The activity of bound HRP was measured using o-phenylmethylsulfonyl fluoride (Sigma-Aldrich). The reactions were stopped by adding 50 μl of 3 M sulfuric acid, and the resultant optical densities at 492 nm (OD492) were measured with an ELISA plate reader.
GM1-ganglioside binding assay.
The affinity of the LTB carried on S. Enteritidis-LTB ghost toward the GM1-ganglioside receptors was determined via a GM1-ELISA. In brief, microtiter ELISA plates were coated with 0.3 μg of monosialoganglioside GM1 (Santa Cruz Biotech) dissolved in PBS buffer and incubated overnight at 4°C. After washing the plates three times in PBST (PBS containing 0.05% Tween 20), the remaining binding sites were blocked by incubating the plates with 1% bovine serum albumin (BSA) (Sigma) in PBS at 37°C for 30 min. The wells were loaded with 100 μl/well of total outer membrane preparation prepared from S. Enteritidis-LTB ghost or S. Enteritidis ghost in phosphate-buffered saline (PBS) and incubated at 37°C for 2 h. The wells were blocked by adding 200 μl/well of 1% bovine serum albumin (BSA) in PBS and incubating at 37°C for 30 min, followed by washing three times with PBST. The wells were then loaded with 100 μl/well of a 1:5,000 dilution of polyclonal anti-LTB rabbit serum diluted in PBS containing 0.1% BSA and incubated at 37°C for 2 h, and subsequently incubated with a 1:8,000 dilution of goat anti-rabbit HRP-conjugated secondary antibody. The activity of bound HRP was measured using o-phenylmethylsulfonyl fluoride (Sigma-Aldrich). The reactions were stopped by adding 50 μl of 3 M sulfuric acid, and the resultant optical densities at 492 nm (OD492) were measured with an ELISA plate reader.
Immunization of chickens.
All experimental work involving animals was approved by the Chonbuk National University Animal Ethics Committee (CBU 2011-0017) and was designed in accordance with the guidelines of the Korean Council on Animal Care. In total, 60 Salmonella-free female brown nick layer chickens were equally divided into three groups: A, B, and C (n = 20 for each). The sterile PBS or ghost preparation was injected in the breast muscle of the chickens. The chickens in group A were used as nonvaccinated controls and were injected intramuscularly with sterile PBS (pH 7.4). Immunization was carried out via the intramuscular route, with 108 ghost cells administered per injection. The chickens receiving immunizations were primed and boosted at 5 and 8 weeks of age, respectively. The chickens in group B were immunized with S. Enteritidis-LTB ghosts, whereas the chickens in group C were immunized with S. Enteritidis ghosts. At 11 weeks of age, the chickens from all groups were challenged with 1 × 109 CFU of the virulent S. Enteritidis strain, JOL1182, via the oral route.
Determination of antibody titers by ELISA.
Titers of S. Enteritidis antigen-specific immunoglobulin G (IgG) and secretory immunoglobulin A (sIgA) antibodies, in addition to LTB-specific IgG titers, were determined by indirect ELISA following immunization. Plasma samples were obtained by centrifuging heparinized blood samples collected from the wing vein, whereas intestinal wash samples were collected using the pilocarpine-based intestinal lavage procedure, as previously described (20). To quantify S. Enteritidis-specific plasma IgG and intestinal sIgA titers, outer membrane protein (OMP) (extracted from S. Enteritidis strain JOL860 cells) was used as the coating antigen. The titers of anti-OMP plasma IgG and intestinal sIgA antibodies were determined by using a chicken IgG and IgA ELISA quantitation kit (Bethyl Laboratories, Montgomery, TX, USA), as previously described (21). To determine LTB-specific plasma IgG titers, purified LTB was used as the coating antigen.
Flow cytometric analysis of T-cell populations.
The T-cell populations in peripheral blood lymphocytes (PBLs) were analyzed 1 week post-booster vaccination. Blood samples were collected from five chickens in each group. Briefly, 5 × 105 cells were incubated with fluorescein isothiocyanate (FITC)-labeled anti-CD3 and biotin-labeled anti-CD4 monoclonal antibodies (SouthernBiotech, Birmingham, AL) for 30 min in the dark at 4°C. For secondary staining of biotin-labeled anti-CD4 molecules, the cells were then incubated with allophycocyanin-labeled streptavidin monoclonal antibodies for 30 min in the dark at 4°C. The stained cells were then washed three times with fluorescence-activated cell sorter (FACS) buffer and resuspended in 0.5 ml PBS. For each sample, 10,000 events were recorded; data analysis was performed with the FlowJo software.
Isolation of the virulent challenge strain from feces.
Fecal samples were collected in 5 ml sterile buffered peptone water (BPW) (Becton, Dickinson and Company, USA), thus generating preenrichment cultures. Ten-fold serial dilutions of preenrichment cultures were plated onto brilliant green agar (BGA) (Becton, Dickinson and Company) medium, and the plates were incubated overnight at 37°C. Samples that were negative for the challenge strain after preenrichment were then subjected to enrichment in Rappaport-Vassiliadis R10 broth (RV) (Becton, Dickinson and Company) through a 48-hour incubation at 42°C. Sterile loops of enriched cultures were then plated onto BGA medium. The number of bacterial colonies obtained without enrichment was determined and expressed as the mean ± standard deviation of the mean log10 CFU/g of feces. Samples that were positive only after enrichment were defined as 1 CFU/g.
Recovery of the challenge strain from internal organs.
Ten chickens from each group were euthanized at 7 or 14 days postchallenge. To determine the bacterial load of the challenge strain in the internal organs, aseptically collected organs were weighed and homogenized in BPW. The numbers of S. Enteritidis CFU per gram of tissue were determined by directly plating 10-fold dilutions of tissue homogenates onto BGA medium. Tissue samples that tested negative after direct plating onto BGA medium were preenriched overnight at 37°C in BPW (1:10) and then enriched in RV broth at 42°C for 48 h. After enrichment, plating loops of RV broth-enriched cultures were streaked onto BGA plates.
Statistical analysis.
Antibody titers, T-cell populations, and fecal and organ CFU are expressed as means ± standard deviation. Mean differences were evaluated with the independent sample t test. Differences were considered statistically significant at a P value of ≤0.05.
RESULTS
Construction of ghost plasmids coexpressing the E lysis gene and eltB.
The plasmid used to generate S. Enteritidis ghosts, pJHL187, harbored the p15A origin of replication, a regulatory ghost cassette, a foreign antigen expression cassette containing the λpR promoter, and the asd gene (Fig. 1). The ghost cassette used here contained the phi X174 phage E lysis gene, located between two face-to-face oriented promoters, a sense λpR promoter, and an antisense ParaBAD promoter (19). This convergent promoter setup has been shown to stably regulate E gene expression when cells are not incubated at the lysis-permissive induction temperature. In the foreign antigen delivery system, the TMD region from the E. coli ompA gene was fused with the His6 epitope and placed upstream from the MCS, into which the eltB gene had been subcloned. The expression of eltB was placed under the control of the λpR promoter and thermosensitive cI857 repressor. The ompA signal peptide directed LTB to be trafficked to the outer membrane.
FIG 1.
Genetic map of plasmid pJHL187-LTB. asd+ p15A origin plasmid carrying the regulatory E lysis ghost cassette and foreign antigen delivery system containing the cloned eltB gene (inset).
Generation and characterization of bacterial ghosts.
Dependence on the asd+ pJHL187-LTB plasmid created a balanced-lethal complementary relationship between JOL1254 cells and pJHL187-LTB. JOL1358 cells were pelleted after attaining mid-logarithmic growth, washed to remove residual l-arabinose, and resuspended in nutrient broth. The cells were then incubated at 42°C, a temperature at which the thermal inactivation of cI857 led to simultaneous induction of LTB expression and E-mediated lysis of JOL1358 cells. The successful induction of cell lysis was confirmed by the lack of further increase in the optical density values at 600 nm (OD600) of the cultures and the lack of CFU obtained from cultures grown at 42°C (data not shown). All 10 replicate experiments exhibited lysis efficiencies of 100% (data not shown). Scanning electron microscopy of S. Enteritidis-LTB ghosts revealed the presence of E-induced transmembrane lysis tunnels; furthermore, ghost cells exhibited a collapsed morphology compared with intact vegetative JOL1358 cells (Fig. 2).
FIG 2.
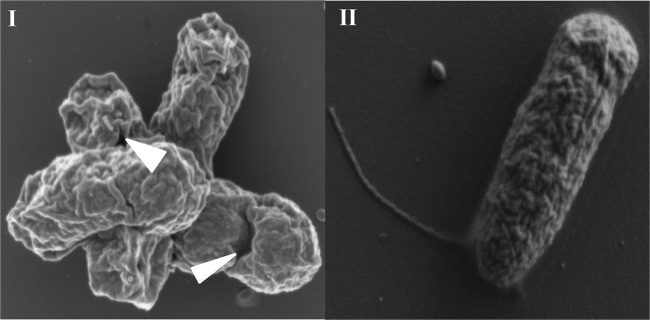
Scanning electron microscopic analysis of S. Enteritidis LTB ghost produced from JOL1358. (I) The lytic action of protein E at 42°C induced the formation of transmembrane lysis tunnels, indicated by the arrowheads. (II) Intact JOL1358 before lysis.
Confirmation of LTB protein expression.
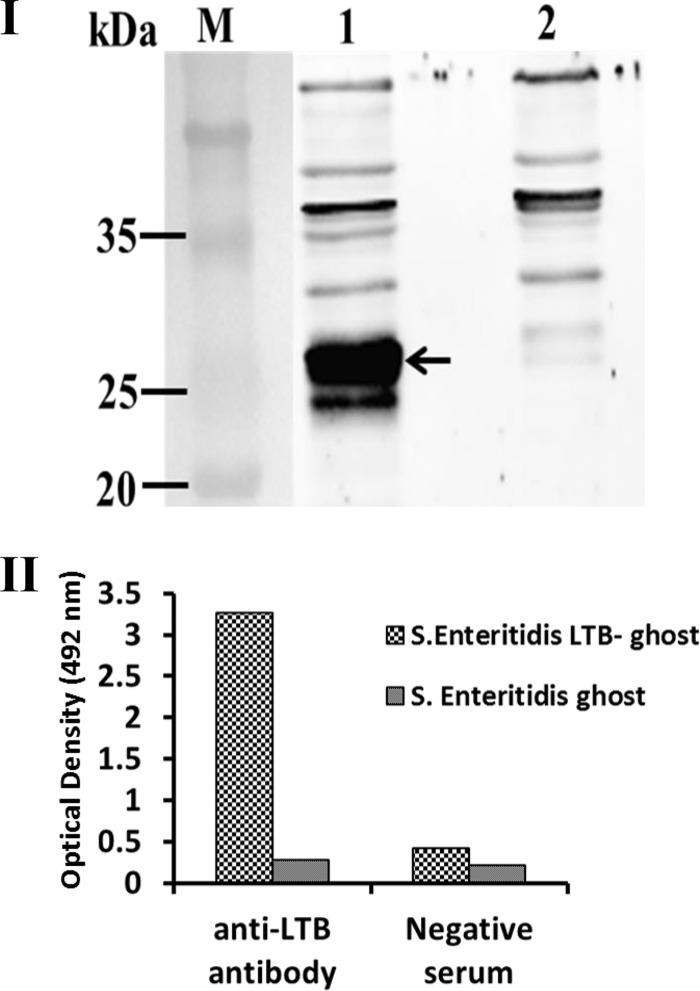
The expression of LTB, induced by isopropyl-β-d-thiogalactopyranoside (IPTG) in BL-21 E. coli cells harboring pET28a-LTB, was detected by Coomassie blue staining of SDS-PAGE gels (data not shown). At 42°C, LTB was expressed by JOL1358 cells, and its fusion with OmpA enabled its transport to the outer membrane. The presence of LTB protein in JOL1358-generated ghosts was detected by ELISA and immunoblotting. The matured OmpA transmembrane (TM) is about 15.3 kDa, and LTB is 11.6 kDa. Thus, the total size of OmpA TM and LTB fusion protein was about 26.9, observed in preparations of S. Enteritidis-LTB ghosts (Fig. 3I). No corresponding band was detected in the negative control. To detect LTB in preparations of S. Enteritidis-LTB ghosts, polyclonal anti-LTB rabbit serum was used as a primary antibody in ELISA. The OD492 values of wells coated with S. Enteritidis-LTB ghosts were approximately 12-fold higher than those of wells coated with S. Enteritidis ghosts, demonstrating the presence of LTB in S. Enteritidis-LTB ghosts (Fig. 3II). Furthermore, wells coated with either S. Enteritidis-LTB ghosts or S. Enteritidis ghosts showed similar OD492 values after incubation with rabbit serum lacking anti-LTB antibodies (Fig. 3II).
FIG 3.
Confirmation of LTB protein expression in S. Enteritidis LTB ghost by immunoblot and ELISA. (I) The LTB protein expressed by S. Enteritidis LTB ghost was detected by immunoblotting with polyclonal anti-LTB rabbit serum. S. Enteritidis ghost only was used as a negative control. Lane M, size marker; lane 1, S. Enteritidis LTB ghost showing LTB-OmpA fusion protein band of size 26.9 kDa (indicated by black arrow); lane 2, S. Enteritidis ghost only. (II) Confirmation of LTB protein expression by ELISA using the polyclonal anti-LTB rabbit serum. A higher OD value was obtained from S. Enteritidis LTB ghost. The S. Enteritidis LTB-ghost and S. Enteritidis ghost showed almost similar OD values when treated with LTB-negative rabbit serum.
GM1-ganglioside binding assay.
The biological function of LTB binding to the GM1-ganglioside receptor was demonstrated by means of a GM1-ganglioside ELISA. As shown in Fig. 4, the LTB expressed in S. Enteritidis-LTB ghost exhibited profound affinity to the GM1 gangliosides. The OD492 values of GM1-ganglioside-coated wells incubated with S. Enteritidis-LTB ghost were approximately 16-fold higher than those of wells incubated with S. Enteritidis ghosts. Thus, the data indicate that the LTB produced in S. Enteritidis-LTB ghost retains its biological functional activity of binding with GM1-ganglioside protein.
FIG 4.
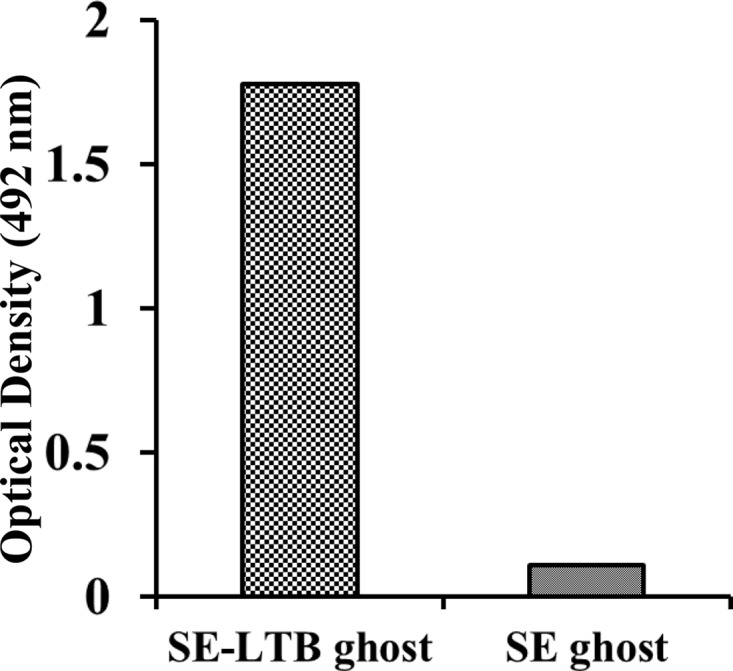
GM1-ELISA measurement for determination of functional activity of LTB protein expressed in the outer membrane of S. Enteritidis-LTB ghost. Outer membrane preparations prepared from S. Enteritidis-LTB ghost or S. Enteritidis ghost was added to the wells coated with GM1 protein and then anti-LTB antiserum was added to identify ganglioside-bound LTB. A higher OD value was obtained from wells coated containing outer membrane preparation of S. Enteritidis-LTB ghost.
Antibody responses in immunized chickens.
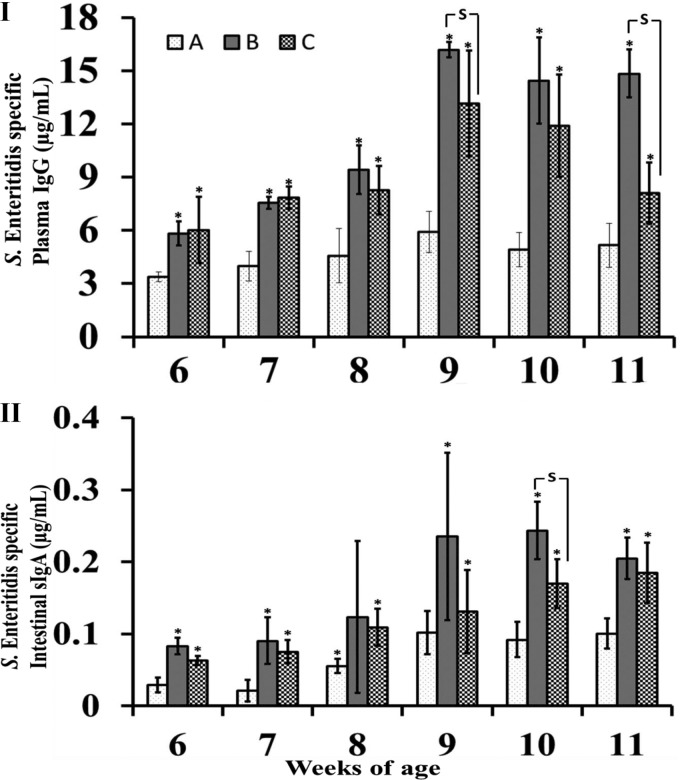
To evaluate the efficacy of S. Enteritidis-LTB ghosts in inducing humoral immune responses, the titers of S. Enteritidis-specific antibodies were measured in both plasma and intestinal wash samples of immunized and nonimmunized chickens. As shown in Fig. 5I, ELISA revealed that the plasma levels of S. Enteritidis-specific antibodies in immunized chickens (groups B and C) were significantly higher than those obtained from nonimmunized chickens in the control group (A) (P ≤ 0.05). The plasma IgG titers of the chickens in group B were higher than those of the chickens in group C, particularly after the booster immunization; these differences were statistically significant at 9 and 11 weeks of age. Antigen-specific sIgA titers were significantly increased in both immunized groups compared with the control group (P ≤ 0.05) (Fig. 5II). Among the immunized groups, the chickens in group B exhibited enhanced the induction of sIgA antibodies compared with the chickens in group C; these differences were statistically significant at 10 weeks of age. In addition, the plasma levels of LTB-specific IgG antibodies of the chickens in group B (immunized with S. Enteritidis-LTB ghosts) were significantly higher than those of the chickens in group A (control group) and the chickens in group C (immunized with S. Enteritidis ghosts) (P ≤ 0.05) (Fig. 6).
FIG 5.
Humoral immune responses. S. Enteritidis-specific humoral immune responses in nonimmunized control (A), S. Enteritidis LTB ghost-immunized (B), and S. Enteritidis ghost-immunized (C) groups were determined at each week postimmunization. (I) Plasma IgG concentrations (μg/ml). (II) Secretory IgA concentrations (μg/ml). Antibody levels are expressed as means ± standard deviation. The asterisks indicate significant differences between the antibody titers of the immunized and nonimmunized groups (P ≤ 0.05). S, significant differences among the vaccinated group, with a P value of ≤ 0.05.
FIG 6.
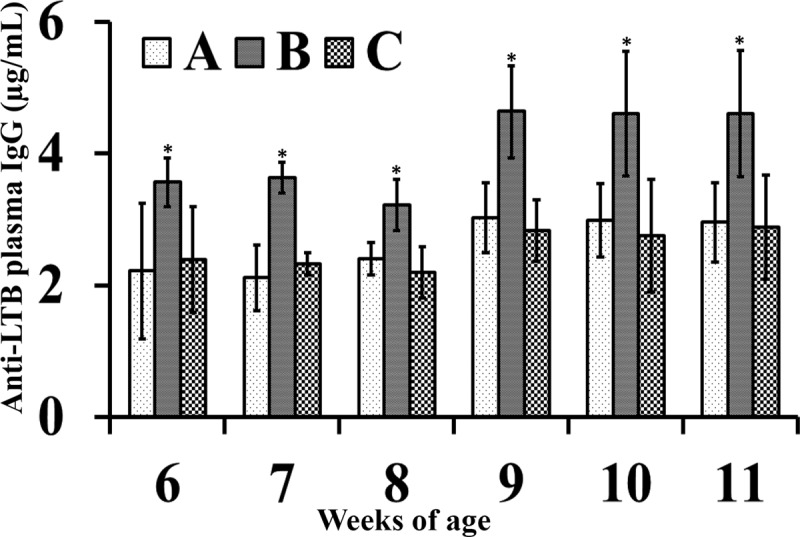
Anti-LTB plasma IgG antibodies. LTB-specific plasma IgG response in nonimmunized control (A), S. Enteritidis LTB ghost-immunized (B) and S. Enteritidis ghost-immunized (C) groups were determined at each week postimmunization. Antibody levels are expressed as mean ± standard deviation. The asterisks indicate significant differences between the antibody titers of the immunized and nonimmunized groups (P ≤ 0.05).
Analysis of postvaccination T-cell subpopulations.
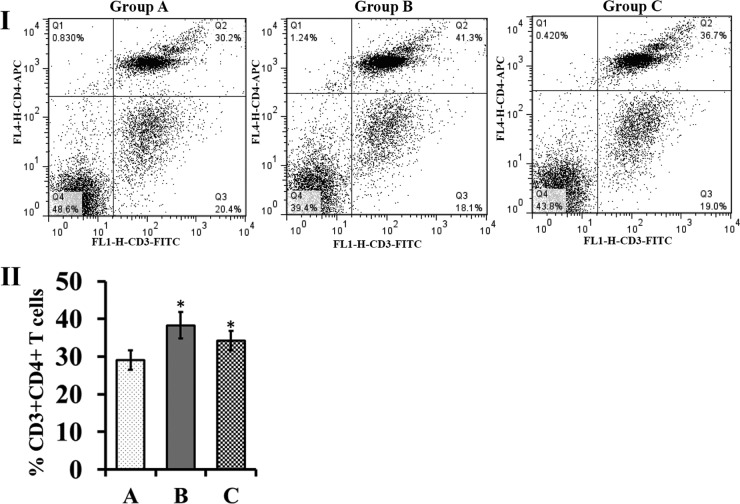
Vaccination of the chickens with S. Enteritidis ghosts induced changes within the populations of peripheral blood CD3+ CD4+ T cells. On the seventh day post-booster vaccination, the amount of peripherally circulating CD3+ CD4+ T cells was significantly higher in both immunized groups than in the control group (P ≤ 0.05) (Fig. 7).
FIG 7.
Flow cytometric analysis of peripheral T-lymphocyte subpopulations after vaccination. (I) Representative dot plots for CD3+ CD4+ T-cell populations of group A, B, and C chickens. The population is represented as a percentage of gated cells. (II) The percentage of peripheral CD3+ CD4+ T cells. The peripheral blood was collected from five randomly selected birds at the 1 week post-booster immunization. The values are shown as means ± standard deviation of five chickens per group. The asterisks indicate significant differences between the T-cell subpopulations of the immunized and nonimmunized groups (*, P ≤ 0.05). Group A, nonimmunized control; group B, S. Enteritidis LTB ghost-immunized chickens; group C, S. Enteritidis ghost-immunized chickens.
Protective efficacy after virulent challenge.
As shown in Table 2, the bacterial load of the challenge strain shed in the feces was higher in the control group than in both immunized groups at both time points tested. The number of fecal samples positive for the challenge strain remained lower in both immunized groups than in the nonimmunized group at all time points. Bacteriological analysis showed that the bacterial loads in the internal organs of the immunized chickens (groups B and C) were also lower than the bacterial loads in the internal organs of the nonimmunized chickens (group A). In the immunized groups (B and C), the bacterial loads of the challenge strain in the liver were significantly lower than those of the nonimmunized control group (A) on the seventh day postchallenge (P ≤ 0.001) (Table 3). An analysis of spleen samples also showed that the bacterial load of the challenge strain was significantly decreased in the immunized groups compared with that in the nonimmunized control group throughout the course of the experiment. The bacterial loads of the challenge strain in the splenic tissue were significantly different between the immunized groups, B and C, on day 7 (P ≤ 0.05). Reduced bacterial loads of the cecal contents of the immunized groups, compared with those of the nonimmunized control group, were observed at both time points; these differences were statistically significant at the seventh day postchallenge (P ≤ 0.05) (Table 3).
TABLE 2.
Fecal shedding of challenge strain detected postchallenge
| Day postchallenge | Groupa | Challenge strain count (mean ± SD) (log10 CFU/g of feces) | No. of positive samplesb |
|---|---|---|---|
| 5 | A | 3.5 ± 1.85 | 4 |
| B | 0.66 ± 1.32c | 1 | |
| C | 1.73 ± 2.15 | 2 | |
| 10 | A | 2.06 ± 1.71 | 3 |
| B | 0 ± 0c | 0c | |
| C | 0 ± 0c | 0c |
Groups: A, nonimmunized challenged control; B, S. Enteritidis LTB-ghost immunized-challenged; C, S. Enteritidis-ghost immunized-challenged.
Number of challenge strain-positive fecal samples out of 5 analyzed samples.
Statistically significant difference between control and immunized groups (P ≤ 0.05).
TABLE 3.
Recovery of challenge strain from internal organs of chickens postchallenge
| Day postchallenge | Groupa | Bacterial recovery (mean ± SD) (log10 CFU/g of tissue)b |
||
|---|---|---|---|---|
| Liver | Spleen | Cecal contents | ||
| 7 | A | 1.46 ± 0.48 | 2.4 ± 0.73 | 4.1 ± 0.69 |
| B | 0.55 ± 0.49** | 0.94 ± 1.0** | 0.87 ± 0.95*** | |
| C | 0.88 ± 0.31** | 1.4 ± 0.49* | 2.5 ± 1.61* | |
| 14 | A | 0.6 ± 0.48 | 1.6 ± 0.65 | 1.45 ± 1.83 |
| B | 0.5 ± 0.5 | 0.55 ± 0.56* | 0.28 ± 0.45 | |
| C | 0.5 ± 0.5 | 1.0 ± 0.0* | 0.14 ± 0.34 | |
Groups are the same as those described in Table 2.
Asterisks indicate the statistically significant differences between control and immunized groups: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
DISCUSSION
The objectives of this study were to: (i) generate S. Enteritidis ghosts carrying LTB and (ii) evaluate the ability of the LTB protein to act as a parenteral adjuvant. Specifically, this study aimed to determine whether the presence of LTB in S. Enteritidis ghosts enhances the immune response generated by and the protective efficacy of the vaccine candidate tested. To generate S. Enteritidis-LTB ghosts, we used a novel plasmid system (Fig. 1) employing: (i) a foreign protein antigen expression cassette designed to facilitate the incorporation of foreign proteins into S. Enteritidis ghosts and (ii) a tightly regulated E-mediated lysis cassette. The λpR-cI857 temperature-sensitive regulatory system alone was inefficient for stable repression of lysis gene E, and it has a drawback of unwanted leaky lysis gene E expression in the absence of induction temperature (19). For stable maintenance of the toxic phi X174 E lysis gene, a tightly regulated ghost cassette was used. In this ghost cassette, the E gene was located in between a sense λpR promoter containing a cI857 regulatory element and an antisense ParaBAD promoter containing an araC regulatory element (19). In the presence of l-arabinose, leaky transcription of lysis gene E at 28°C from the sense λpR promoter can be repressed by an antisense RNA simultaneously expressed from the ParaBAD promoter (19). In our foreign antigen delivery construct, expression was induced in a temperature-dependent manner via the λpR promoter; furthermore, the thermolabile cI857 repressor was used to regulate the expression of the foreign protein antigen. This thermoregulated expression system has been well characterized and successfully used to produce many recombinant proteins. This system employs a strong and tightly regulated promoter; furthermore, this system does not require the use of special medium, toxic inducers, or expensive chemical inducers (22). In our construct, the 5′ region of the E. coli ompA gene was cloned under the control of the λpR system. This region of ompA encodes the N-terminal amino acid residues, which integrate into the membrane as a β-barrel (18). This signal peptide sequence (MKKTAIAIAVALAGFATVAQA) serves as a sorting signal to direct the trafficking of OmpA to the outer membrane (23).
We cloned the eltB gene into the MCS such that eltB was fused with the ompA signal sequence so the fusion protein would be expressed under the control of the λpR promoter. In the absence of arabinose, shifting JOL1358 cultures from 28°C to 42°C triggers λpR-driven expression of eltB (a foreign antigen delivery system promoter) and λpR-driven expression of the E lysis gene (a regulatory ghost cassette promoter). The fusion of LTB with the ompA signal peptide sequence directs the translocation of the resultant LTB fusion protein to the S. Enteritidis outer membrane (17). In this study, the simultaneous expression of LTB and E proteins generated S. Enteritidis-LTB ghosts. Before the induction of lysis, JOL1358 cells were present in the distinct vegetative form and exhibited normal morphology (Fig. 2II). The expression of E protein led to fusion of the inner and outer membranes of the bacterial cell envelope, thereby generating transmembrane lysis tunnels (Fig. 2I). After observation of SEM images of S. Enteritidis-LTB ghosts, we concluded that lysis tunnels exhibit an irregular shape; this irregularity might be due to the fact that transmembrane tunnels do not exhibit a rigid fixed structure (24). Since the driving force for the rapid evacuation of cytoplasmic contents is the osmotic pressure difference between the cytoplasm and the surrounding culture medium, this pressure difference leads to the shrinkage of the bacterial envelope; thus, S. Enteritidis-LTB ghosts appear to be collapsed (Fig. 2I). Expression of the LTB protein was confirmed by ELISA and an immunoblot assay (Fig. 3). As indicated by the results of the GM1-ganglioside binding assay (Fig. 4), the LTB protein expressed by S. Enteritidis-LTB ghost is functionally active and might bind with GM1 receptors present on eukaryotic cells to demonstrate adjuvant activity. The expression of LTB in the outer membrane of the bacterial ghosts may enhance the uptake of ghost antigens through the binding of LTB to cell surface GM1 receptors (25). Chemical coupling (26) or genetic fusion (27) of antigens with LTB has been shown to enhance the induction of both mucosal and systemic immune responses. The highly improved adjuvanticity of LTB when coupled to antigens is thought to be due to the efficient presentation of the coupled antigen by antigen-presenting cells (APCs) (14). The action of the LTB protein as a mucosal adjuvant is widely accepted (14). Although the bacterial ghost used in the present study is genetically coupled with the LTB protein, we opted for a parenteral route of immunization instead of an oral route. Because the lower immune response elicited following oral delivery of nonreplicating vaccine antigens is frequently observed in chickens, this might be caused by the degradation of antigenic material by digestive enzymes of the gastrointestinal tract. Therefore, only a small amount of fully immunogenic material can reach the gut-associated lymphoid tissue (GALT), which may result in lower induction of the immune response (28). The parenteral adjuvant activity of LTB has been evaluated in subcutaneous (29), intramuscular (30), intraperitoneal (31), intravenous (32), intradermal (33), and transcutaneous (34) immunizations. Furthermore, the version of LTB in our construct lacks the toxic portion of this molecule and therefore can be used as a safe adjuvant (35).
After infection via the oral route, Salmonella rapidly crosses the intestinal mucosa and penetrates the deeper tissues, mainly the splenic and liver tissues. The ability of Salmonella to infect a variety of phagocytic and nonphagocytic cells leads to massive bacterial replication, resulting in a high bacterial load in its target organs (36). Eventually, successful establishment of the acquired immune response after immunization is required to control and eradicate the bacteria, thus conferring protection against virulent infection (37). Immunization with S. Enteritidis ghosts is known to stimulate mucosal and systemic humoral immunity by inducing strong anti-Salmonella intestinal sIgA and plasma IgG antibody responses (10). Systemic antibodies participate in protection during the early stages of infection, during which S. Enteritidis resides extracellularly; these molecules act by inactivating S. Enteritidis before it penetrates its target cells (38). The interaction of systemic antibodies with S. Enteritidis results in its opsonization, thus enhancing its receptor-mediated uptake by macrophages. In this study, the immunized chickens (groups B and C) exhibited significantly increased titers of S. Enteritidis-specific plasma IgG antibodies compared with those from the control chickens (group A) (Fig. 5I). The chickens in group B exhibited enhanced induction of the systemic antibody response compared with the chickens in group C, indicating that the presence of LTB may have facilitated increased uptake of antigen by APCs, thus leading to superior induction of IgG antibodies (39). In natural infection, host animals are infected via the oral route by ingesting food contaminated with S. Enteritidis (36). Importantly, sIgA molecules present in the intestinal mucus act as an immunological barrier and have been proposed to prevent the adherence of Salmonella organisms to the intestinal lining, consequently preventing Salmonella penetration into deeper tissues (40). The intestinal sIgA titers from the chickens in the immunized groups (B and C) were significantly higher than those from the chickens in the control group (A) (Fig. 5II). The chickens in group B showed enhanced induction of sIgA antibodies compared with the chickens in group C, indicating that parenteral administration of antigens coupled with LTB enhances mucosal immune responses. The chickens immunized with S. Enteritidis-LTB ghosts also showed significant induction of LTB-specific antibodies (Fig. 6), indicating that the LTB present in the outer membrane of the S. Enteritidis ghosts was functionally stable.
Since Salmonella is also capable of residing and multiplying intracellularly, cell-mediated immune responses also play a major role in protection against infection (36, 37). In this study, both immunized groups showed significantly elevated populations of CD3+ CD4+ T cells (Fig. 7). CD4+ T cells are particularly important for establishing the acquired immune response against Salmonella (41) by producing macrophage-activating cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). CD4+ T cells also assist B cells in antibody production (42). In the present study, no difference was observed in the overall peripheral CD3+ CD4+ T-cell population of the immunized group B and C chickens. This increase in CD4+ T cells might be due to the presence of S. Enteritidis ghost antigen in both immunization regimens. However, elucidation of the specific role of the LTB protein for augmentation of functional CD4 T-cell responses when it is coupled with S. Enteritidis ghost antigen is required to gain further knowledge on this subject.
Vaccination with bacterial ghosts protects hosts against virulent Gram-negative infections (11, 43). Here, we demonstrated the efficacy of vaccination with S. Enteritidis-LTB ghosts and demonstrated that the immunized chickens were protected against virulent challenge. The reduced fecal shedding of the challenge strain from the immunized groups might be due to protective immunity, since nonimmunized chickens shed high levels of the challenge strain. Furthermore, the higher bacterial loads of the challenge strain recovered from the liver, spleen, and cecal contents of the nonimmunized chickens indicate that they were highly susceptible to virulent S. Enteritidis infection. Compared with the nonimmunized group, the bacterial loads of the challenge strain in the organs of the immunized chickens were significantly lower (Table 3), indicating that the protective immunity induced by vaccination conferred resistance to virulent infection for chickens, particularly those in group B (36). The protection was also observed in group C, but to a lesser extent.
In conclusion, this study reports a unique vaccination strategy involving the expression of LTB in the outer membrane of S. Enteritidis ghosts. This incorporation of LTB into S. Enteritidis ghost preparations led to superior induction of antigen-specific immune responses and protects chickens against virulent S. Enteritidis challenge.
ACKNOWLEDGMENT
This work was supported by the National Research Foundation of Korea (NRF), funded by the Korean government (MISP) (grant 2013R1A4A1069486).
Footnotes
Published ahead of print 26 March 2014
REFERENCES
- 1.Howard ZR, O'Bryan CA, Crandall PG, Ricke SC. 2012. Salmonella Enteritidis in shell eggs: current issues and prospects for control. Food. Res. Int. 45:755–764. 10.1016/j.foodres.2011.04.030 [DOI] [Google Scholar]
- 2.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM, International Collaboration on Enteric Disease ‘Burden of Illness' Studies 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50:882–889. 10.1086/650733 [DOI] [PubMed] [Google Scholar]
- 3.Thomas ME, Klinkenberg D, Ejeta G, Van Knapen F, Bergwerff AA, Stegeman JA, Bouma A. 2009. Quantification of horizontal transmission of Salmonella enterica serovar Enteritidis bacteria in pair-housed groups of laying hens. Appl. Environ. Microbiol. 75:6361–6366. 10.1128/AEM.00961-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penha Filho RA, de Paiva JB, da Silva MD, de Almeida AM, Berchieri A., Jr 2010. Control of Salmonella Enteritidis and Salmonella Gallinarum in birds by using live vaccine candidate containing attenuated Salmonella Gallinarum mutant strain. Vaccine 28:2853–2859. 10.1016/j.vaccine.2010.01.058 [DOI] [PubMed] [Google Scholar]
- 5.Barrow PA. 2007. Salmonella infections: immune and non-immune protection with vaccines. Avian Pathol. 36:1–13. 10.1080/03079450601113167 [DOI] [PubMed] [Google Scholar]
- 6.Pace JL, Rossi HA, Esposito VM, Frey SM, Tucker KD, Walker RI. 1998. Inactivated whole-cell bacterial vaccines: current status and novel strategies. Vaccine 16:1563–1574. 10.1016/S0264-410X(98)00046-2 [DOI] [PubMed] [Google Scholar]
- 7.Meeusen ENT, Walker J, Peters A, Pastoret PP, Jungersen G. 2007. Current status of veterinary vaccines. Clin. Microbiol. Rev. 20:489. 10.1128/CMR.00005-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jalava K, Hensel A, Szostak M, Resch S, Lubitz W. 2002. Bacterial ghosts as vaccine candidates for veterinary applications. J. Control Release 85:17–25 [DOI] [PubMed] [Google Scholar]
- 9.Peng W, Si W, Yin L, Liu H, Yu S, Liu S, Wang C, Chang Y, Zhang Z, Hu S, Du Y. 2011. Salmonella Enteritidis ghost vaccine induces effective protection against lethal challenge in specific-pathogen-free chicks. Immunobiology 216:558–565. 10.1016/j.imbio.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 10.Jawale CV, Chaudhari AA, Jeon BW, Nandre RM, Lee JH. 2012. Characterization of a novel inactivated Salmonella enterica serovar Enteritidis vaccine candidate generated using a modified cI857/λ PR/gene E expression system. Infect. Immun. 80:1502. 10.1128/IAI.06264-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhari AA, Jawale CV, Kim SW, Lee JH. 2012. Construction of a Salmonella Gallinarum ghost as a novel inactivated vaccine candidate and its protective efficacy against fowl typhoid in chickens. Vet. Res. 43:44. 10.1186/1297-9716-43-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekong EE, Okenu DN, Mania-Pramanik J, He Q, Igietseme JU, Ananaba GA, Lyn D, Black C, Eko FO. 2009. A Vibrio cholerae ghost-based subunit vaccine induces cross-protective chlamydial immunity that is enhanced by CTA2B, the nontoxic derivative of cholera toxin. FEMS Immunol. Med. Microbiol. 55:280–291. 10.1111/j.1574-695X.2008.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sixma TK, Pronk SE, Kalk KH, Wartna ES, van Zanten BA, Witholt B, Hol WG. 1991. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature 351:371–377. 10.1038/351371a0 [DOI] [PubMed] [Google Scholar]
- 14.Freytag LC, Clements JD. 2005. Mucosal adjuvants. Vaccine 23:1804–1813. 10.1016/j.vaccine.2004.11.010 [DOI] [PubMed] [Google Scholar]
- 15.Czerkinsky C, Russell MW, Lycke N, Lindblad M, Holmgren J. 1989. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect. Immun. 57:1072–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Dowd AM, Botting CH, Precious B, Shawcross R, Randall RE. 1999. Novel modifications to the C-terminus of LTB that facilitate site-directed chemical coupling of antigens and the development of LTB as a carrier for mucosal vaccines. Vaccine 17:1442–1453. 10.1016/S0264-410X(98)00375-2 [DOI] [PubMed] [Google Scholar]
- 17.Singh SP, Williams YU, Miller S, Nikaido H. 2003. The C-terminal domain of Salmonella enterica serovar Typhimurium OmpA is an immunodominant antigen in mice but appears to be only partially exposed on the bacterial cell surface. Infect. Immun. 71:3937–3946. 10.1128/IAI.71.7.3937-3946.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arora A, Rinehart D, Szabo G, Tamm LK. 2000. Refolded outer membrane protein A of Escherichia coli forms ion channels with two conductance states in planar lipid bilayers. J. Biol. Chem. 275:1594–1600. 10.1074/jbc.275.3.1594 [DOI] [PubMed] [Google Scholar]
- 19.Jawale CV, Kim SW, Lee JH. 2014. Tightly regulated bacteriolysis for production of empty Salmonella Enteritidis envelope. Vet. Microbiol. 169:179–187. 10.1016/j.vetmic.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 20.Matsuda K, Chaudhari AA, Kim SW, Lee KM, Lee JH. 2010. Physiology, pathogenicity and immunogenicity of lon and/or cpxR deleted mutants of Salmonella Gallinarum as vaccine candidates for fowl typhoid. Vet. Res. 41:59. 10.1051/vetres/2010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nandre RM, Chaudhari AA, Matsuda K, Lee JH. 2011. Immunogenicity of a Salmonella Enteritidis mutant as vaccine candidate and its protective efficacy against salmonellosis in chickens. Vet. Immunol. Immunopathol. 144:299–311. 10.1016/j.vetimm.2011.08.015 [DOI] [PubMed] [Google Scholar]
- 22.Makrides SC. 1996. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol. Rev. 60:512–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjöström M, Wold S, Wieslander A, Rilfors L. 1987. Signal peptide amino acid sequences in Escherichia coli contain information related to final protein localization. A multivariate data analysis. EMBO J. 6:823–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witte A, Wanner G, Bläsi U, Halfmann G, Szostak M, Lubitz W. 1990. Endogenous transmembrane tunnel formation mediated by phi X174 lysis protein E. J. Bacteriol. 172:4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moyle PM, McGeary RP, Blanchfield JT, Toth I. 2004. Mucosal immunisation: adjuvants and delivery systems. Curr. Drug Deliv. 1:385–396. 10.2174/1567201043334588 [DOI] [PubMed] [Google Scholar]
- 26.Weltzin R, Guy B, Thomas WD, Jr, Giannasca PJ, Monathi TP. 2000. Parenteral adjuvant activities of Escherichia coli heat-labile toxin and its B subunit for immunization of mice against gastric Helicobacter pylori infection. Infect. Immun. 68:2775–2782. 10.1128/IAI.68.5.2775-2782.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin M, Hajishengallis G, Metzger DJ, Michalek SM, Connell TD, Russell MW. 2001. Recombinant antigen-enterotoxin A2/B chimeric mucosal immunogens differentially enhance antibody responses and B7-dependent costimulation of CD4+ T cells. Infect. Immun. 69:252–261. 10.1128/IAI.69.1.252-261.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muir WI, Bryden WL, Husband AJ. 2000. Immunity, vaccination and the avian intestinal tract. Dev. Comp. Immunol. 24:325–342. 10.1016/S0145-305X(99)00081-6 [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto S, Takeda Y, Yamamoto M, Kurazono H, Imaoka K, Yamamoto M, Fujihashi K, Noda M, Kiyono H, McGhee JR. 1997. Mutants in the ADP-ribosyltransferase cleft of cholera toxin take diarrheagenicity but retain adjuvanticity. J. Exp. Med. 185:1203–1210. 10.1084/jem.185.7.1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conceição FR, Moreira AN, Dellagostin OA. 2006. A recombinant chimera composed of R1 repeat region of Mycoplasma hyopneumoniae P97 adhesin with Escherichia coli heat-labile enterotoxin B subunit elicits immune response in mice. Vaccine 24:5734–5743. 10.1016/j.vaccine.2006.04.036 [DOI] [PubMed] [Google Scholar]
- 31.Agren LC, Ekman L, Löwenadler B, Nedrud JG, Lycke NY. 1999. Adjuvanticity of the cholera toxin A1-based gene protein, CTA1-DD, is critically dependent on the ADP-ribosyltransferase and Ig-binding activity. J. Immunol. 162:2432–2440 [PubMed] [Google Scholar]
- 32.Hornquist E, Lycke N. 1993. Cholera toxin adjuvant greatly promotes antigen printing of T cells. Eur. J. Immunol. 23:2136–2143. 10.1002/eji.1830230914 [DOI] [PubMed] [Google Scholar]
- 33.Akhiani AA, Nilsson LA, Ouchterlony O. 1993. Effect of cholera toxin on vaccine-induced immunity and infection in murine schistosomiasis mansoni. Infect. Immun. 61:4919–4924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frankenburg S, Grinberg I, Bazak Z, Fingerut L, Pitcovski J, Gorodetsky, Peretz T, Spira RM, Skornik Y, Goldstein RS. 2007. Immunological activation following transcutaneous delivery of HR-gp100 protein. Vaccine 25:4564–4570. 10.1016/j.vaccine.2007.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fingerut E, Gutter B, Goldway M, Eliahoo D, Pitcovski J. 2006. B subunit of E. coli enterotoxin as adjuvant and carrier in oral and skin vaccination. Vet. Immunol. Immunopathol. 112:253–263. 10.1016/j.vetimm.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 36.Chappell L, Kaiser P, Barrow P, Jones MA, Johnston C, Wigley P. 2009. The immunobiology of avian systemic salmonellosis. Vet. Immunol. Immunopathol. 128:53–59. 10.1016/j.vetimm.2008.10.295 [DOI] [PubMed] [Google Scholar]
- 37.Mastroeni P, Ménager N. 2003. Development of acquired immunity to Salmonella. J. Med. Microbiol. 52:453–459. 10.1099/jmm.0.05173-0 [DOI] [PubMed] [Google Scholar]
- 38.Collins FM. 1974. Vaccines and cell-mediated immunity. Bacteriol. Rev. 38:371–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hur J, Lee JH. 2011. Enhancement of immune responses by an attenuated Salmonella enterica serovar Typhimurium strain secreting an Escherichia coli heat-labile enterotoxin B subunit protein as an adjuvant for a live Salmonella vaccine candidate. Clin. Vaccine Immunol. 18:203–209. 10.1128/CVI.00407-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasetti MF, Simon JK, Sztein MB, Levine MM. 2011. Immunology of gut mucosal vaccines. Immunol. Rev. 239:125–148. 10.1111/j.1600-065X.2010.00970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittrücker HW, Kaufmann SHE. 2000. Immune response to infection with Salmonella Typhimurium in mice. J. Leukoc. Biol. 67:457–463 [DOI] [PubMed] [Google Scholar]
- 42.McSorley SJ, Cookson BT, Jenkins MK. 2000. Characterization of CD4+ T cell responses during natural infection with Salmonella Typhimurium. J. Immunol. 164:986–993 [DOI] [PubMed] [Google Scholar]
- 43.Hu M, Zhang Y, Xie F, Li G, Li J, Si W, Liu S, Hu S, Zhang Z, Shen N, Wang C. 2013. Protection of piglets by a Haemophilus parasuis ghost vaccine against homologous challenge. Clin. Vaccine Immunol. 20:795–802. 10.1128/CVI.00676-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang HY, Srinivasan J, Curtiss R., III 2002. Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infect. Immun. 70:1739–1749. 10.1128/IAI.70.4.1739-1749.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]